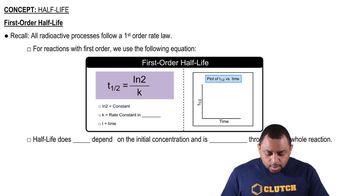
Textbook Question
Bars of iron are put into each of the three beakers as shown here. In which beaker—A, B, or C—would you expect the iron to show the most corrosion ? [Section 20.8]


 Verified step by step guidance
Verified step by step guidance

Bars of iron are put into each of the three beakers as shown here. In which beaker—A, B, or C—would you expect the iron to show the most corrosion ? [Section 20.8]
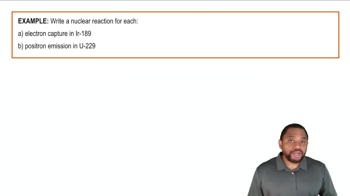
Magnesium, the element, is produced commercially by electrolysis from a molten salt (the 'electrolyte') using a cell similar to the one shown here. (a) What is the most common oxidation number for Mg when it is part of a salt?
Magnesium, the element, is produced commercially by electrolysis from a molten salt (the 'electrolyte') using a cell similar to the one shown here. (b) Chlorine gas is evolved as voltage is applied in the cell. Knowing this, identify the electrolyte.
(b) On which side of an oxidation half-reaction do the electrons appear?