For each of the following reactions, write a balanced equation, calculate the standard emf, calculate ∆G° at 298 K, and calculate the equilibrium constant K at 298 K. (b) In acidic solution, copper(I) ion is oxidized to copper(II) ion by nitrate ion.
Ch.20 - Electrochemistry

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 20, Problem 54
If the equilibrium constant for a one-electron redox reaction at 298 K is 8.7 * 10^4, calculate the corresponding ∆G° and E°.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Recall the relationship between the equilibrium constant (K) and the standard Gibbs free energy change (∆G°) using the equation: ∆G° = -RT ln(K), where R is the universal gas constant (8.314 J/mol·K) and T is the temperature in Kelvin.
Step 2: Substitute the given values into the equation: R = 8.314 J/mol·K, T = 298 K, and K = 8.7 * 10^4. Calculate the natural logarithm of K, ln(8.7 * 10^4).
Step 3: Calculate ∆G° by substituting the values of R, T, and ln(K) into the equation ∆G° = -RT ln(K).
Step 4: Use the relationship between ∆G° and the standard cell potential (E°) for a redox reaction: ∆G° = -nFE°, where n is the number of moles of electrons transferred (n = 1 for a one-electron reaction) and F is the Faraday constant (96485 C/mol).
Step 5: Rearrange the equation to solve for E°: E° = -∆G°/(nF). Substitute the calculated value of ∆G° and the constants n and F to find E°.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Equilibrium Constant (K)
The equilibrium constant (K) quantifies the ratio of the concentrations of products to reactants at equilibrium for a reversible reaction. In redox reactions, a higher K value indicates a greater tendency for the reaction to proceed in the forward direction, favoring product formation. This constant is crucial for calculating thermodynamic properties like Gibbs free energy and cell potential.
Recommended video:
Guided course

Equilibrium Constant K
Gibbs Free Energy (∆G°)
Gibbs free energy (∆G°) is a thermodynamic potential that indicates the spontaneity of a reaction at constant temperature and pressure. It is related to the equilibrium constant by the equation ∆G° = -RT ln(K), where R is the universal gas constant and T is the temperature in Kelvin. A negative ∆G° suggests that the reaction is spontaneous under standard conditions.
Recommended video:
Guided course

Gibbs Free Energy of Reactions
Electrode Potential (E°)
Electrode potential (E°) is a measure of the tendency of a chemical species to be reduced, expressed in volts. It is related to the Gibbs free energy change by the equation ∆G° = -nFE°, where n is the number of moles of electrons transferred and F is Faraday's constant. A positive E° indicates a favorable reaction, while a negative E° suggests non-spontaneity.
Recommended video:
Guided course
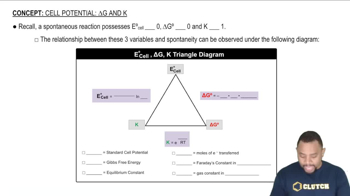
Relationship between ∆E°, ∆G°, and K
Related Practice
Textbook Question
Textbook Question
For each of the following reactions, write a balanced equation, calculate the standard emf, calculate ∆G° at 298 K, and calculate the equilibrium constant K at 298 K. (c) In basic solution, Cr1OH231s2 is oxidized to CrO42-1aq2 by ClO-1aq2.
Textbook Question
Using the standard reduction potentials listed in Appendix E, calculate the equilibrium constant for each of the following reactions at 298 K:
(a) Fe(s) + Ni2+(aq) → Fe2+(aq) + Ni(s)
(b) Co(s) + 2 H+(aq) → Co2+(aq) + H2(g)
(c) 10 Br-(aq) + 2 MnO4-(aq) + 16 H+(aq) → 2 Mn2+(aq) + 8 H2O(l) + 5 Br2(l)
Textbook Question
A cell has a standard cell potential of +0.177 V at 298 K. What is the value of the equilibrium constant for the reaction
(a) if n = 1?
1
views
