Complete and balance the following equations, and identify the oxidizing and reducing agents: (a) Cr2O72-(aq) + I-(aq) → Cr3+(aq) + IO3-(aq) (acidic solution) (b) MnO4-(aq) + CH3O(1aq) → Mn2+(aq) + HCOOH(aq) (acidic solution) (c) I2(s) + OCl-(aq) → IO3-(aq) + Cl-(aq) (acidic solution)
Ch.20 - Electrochemistry

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 20, Problem 26a,b,c,d
Complete and balance the following equations, and identify the oxidizing and reducing agents. (Recall that the O atoms in hydrogen peroxide, H2O2, have an atypical oxidation state.) (a) NO2-(aq) + Cr2O72-(aq) → Cr3+(aq) + NO3-(aq) (acidic solution) (b) S(s) + HNO3(aq) → H2SO3(aq) + N2O(g) (acidic solution) (c) Cr2O72- (aq) + CH3OH(aq) → HCOOH(aq) + Cr3+(aq) (acidic solution) (d) BrO3-(aq) + N2H4(g) → Br-(aq) + N2(g) (acidic solution)
 Verified step by step guidance
Verified step by step guidance1
Identify the oxidation states of each element in the reactants and products. For example, in \( \text{Cr}_2\text{O}_7^{2-} \), chromium has an oxidation state of +6, and in \( \text{Cr}^{3+} \), it is +3.
Determine which elements are oxidized and which are reduced by comparing the changes in oxidation states. Oxidation involves an increase in oxidation state, while reduction involves a decrease.
Write the half-reactions for the oxidation and reduction processes. For example, the reduction half-reaction involves \( \text{Cr}_2\text{O}_7^{2-} \) being reduced to \( \text{Cr}^{3+} \).
Balance each half-reaction for mass and charge. In acidic solutions, add \( \text{H}^+ \) ions to balance hydrogen and \( \text{H}_2\text{O} \) to balance oxygen.
Combine the balanced half-reactions, ensuring that the electrons lost in the oxidation half-reaction equal the electrons gained in the reduction half-reaction. Identify the oxidizing agent (the species that is reduced) and the reducing agent (the species that is oxidized).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
8mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Oxidation and Reduction
Oxidation and reduction are chemical processes that involve the transfer of electrons between substances. Oxidation refers to the loss of electrons, resulting in an increase in oxidation state, while reduction involves the gain of electrons, leading to a decrease in oxidation state. In redox reactions, one species is oxidized and another is reduced, which is essential for balancing chemical equations.
Recommended video:
Guided course
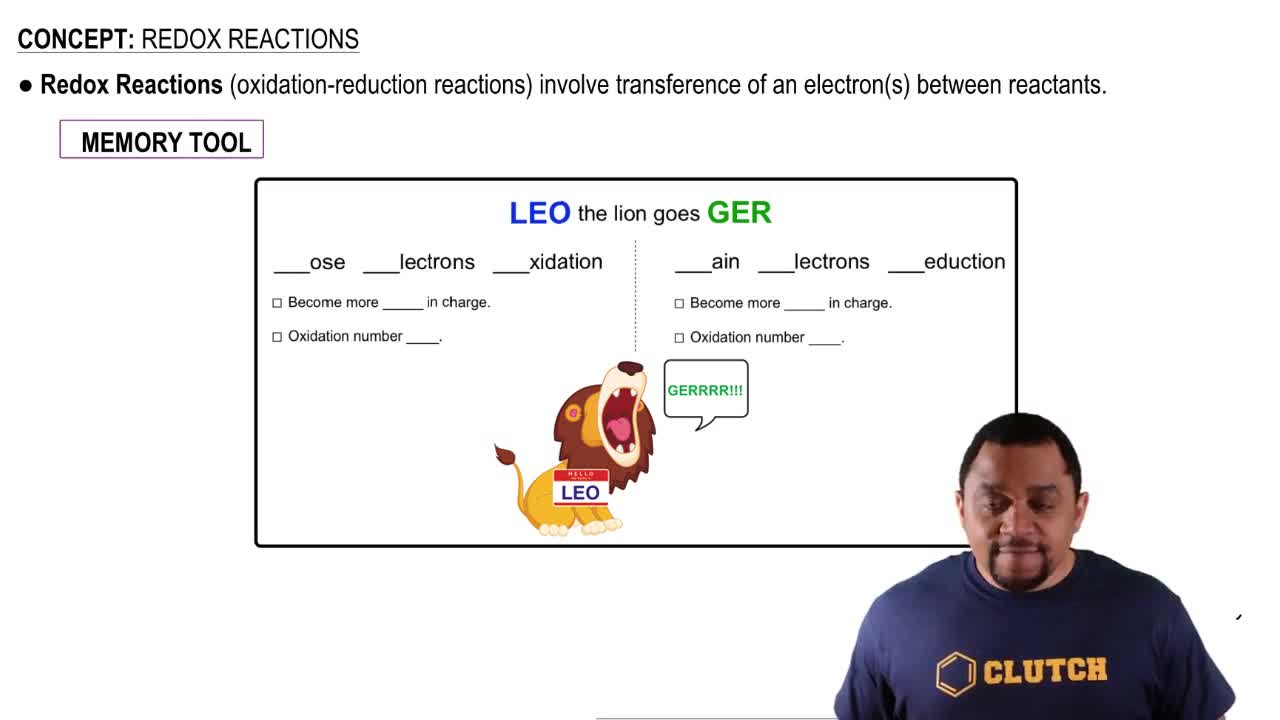
Oxidation and Reduction Reactions
Balancing Redox Reactions
Balancing redox reactions requires ensuring that both mass and charge are conserved. This often involves separating the reaction into half-reactions for oxidation and reduction, balancing each half for atoms and charge, and then combining them to form the overall balanced equation. In acidic solutions, hydrogen ions (H+) and water (H2O) are commonly used to achieve balance.
Recommended video:
Guided course

Balancing Basic Redox Reactions
Identifying Oxidizing and Reducing Agents
In a redox reaction, the oxidizing agent is the substance that gains electrons and is reduced, while the reducing agent is the substance that loses electrons and is oxidized. Identifying these agents involves analyzing the changes in oxidation states of the elements involved in the reaction. This understanding is crucial for determining the roles of different reactants in the overall chemical process.
Recommended video:
Guided course

Oxidizing and Reducing Agents
Related Practice
Textbook Question
Textbook Question
Complete and balance the following equations, and identify the oxidizing and reducing agents: As2O3(s) + NO3-(aq) → H3AsO4(aq) + N2O3(aq) (acidic solution)
1
views
Textbook Question
Complete and balance the following equations, and identify the oxidizing and reducing agents: MnO4-(aq) + Br-(aq) → MnO2(s) + BrO3-(aq) (basic solution)
Textbook Question
Complete and balance the following equations, and identify the oxidizing and reducing agents. (Recall that the O atoms in hydrogen peroxide, H2O2, have an atypical oxidation state.) H2O21aq2 + ClO21aq2 ¡ ClO2-1aq2 + O21g2 (basic solution)
