Textbook Question
From each of the following pairs of substances, use data in Appendix E to choose the one that is the stronger reducing agent: (d) BrO3-1aq2 or IO3-1aq2

 Verified step by step guidance
Verified step by step guidance
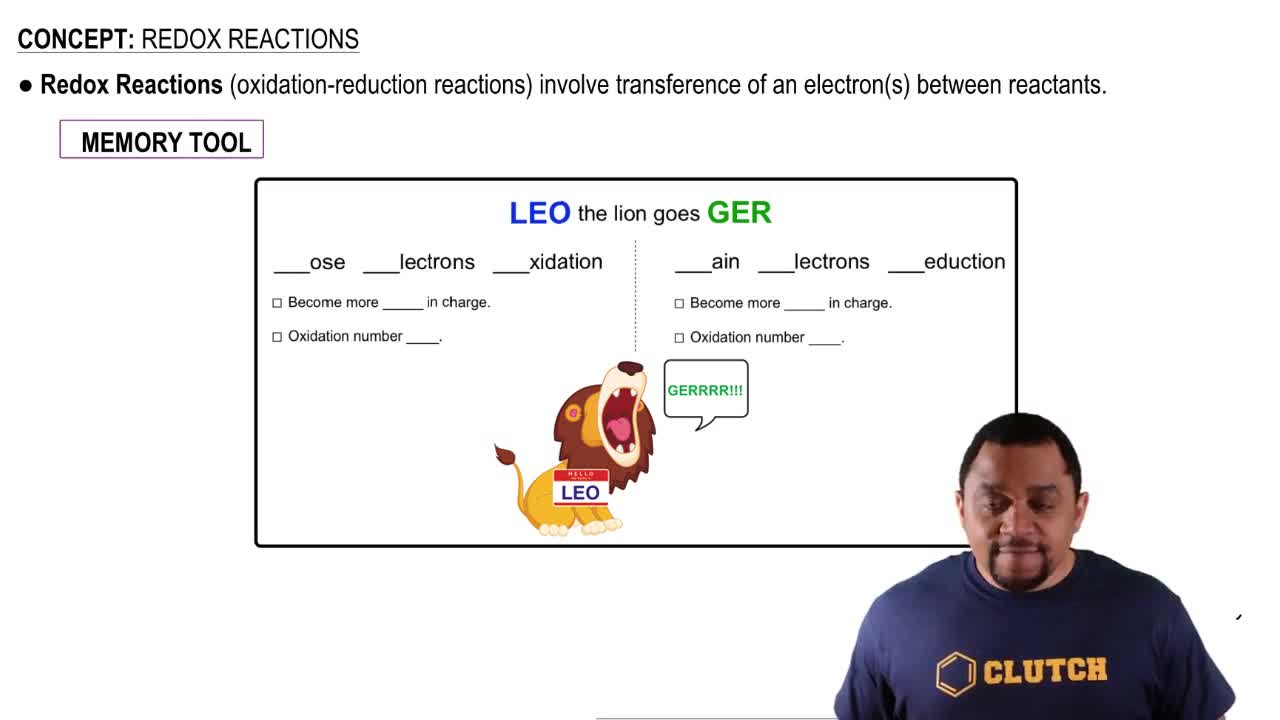

From each of the following pairs of substances, use data in Appendix E to choose the one that is the stronger reducing agent: (d) BrO3-1aq2 or IO3-1aq2
By using the data in Appendix E, determine whether each of the following substances is likely to serve as an oxidant or a reductant: (a) Cl2(g), (b) MnO4- (aq, acidic solution), (c) Ba(s)
Is each of the following substances likely to serve as an oxidant or a reductant: (a) Ce3+(aq) (b) Ca(s) (c) ClO3-(aq) (d) N2O5(g)?
(a) Assuming standard conditions, arrange the following in order of increasing strength as oxidizing agents in acidic solution: Cr2O72-, H2O2, Cu2+, Cl2, O2.
(b) Arrange the following in order of increasing strength as reducing agents in acidic solution: Zn, I-, Sn2+, H2O2, Al.