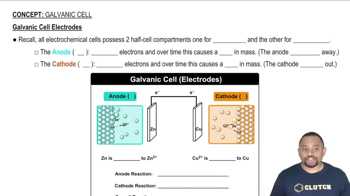
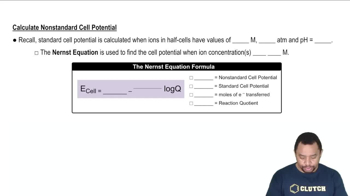
Textbook Question
A voltaic cell is constructed that uses the following half-cell reactions:
Cu+(aq) + e- → Cu(s)
I2(s) + 2 e- → 2 I-(aq)
The cell is operated at 298 K with [Cu+] = 0.25 M and [I-] = 0.035 M.
(a) Determine E for the cell at these concentrations.