A voltaic cell that uses the reaction PdCl42-(aq) + Cd(s) → Pd(s) + 4 Cl-(aq) + Cd2+(aq) has a measured standard cell potential of +1.03 V. (a) Write the two half-cell reactions.
Ch.20 - Electrochemistry

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 20, Problem 36c
A voltaic cell that uses the reaction PdCl42-(aq) + Cd(s) → Pd(s) + 4 Cl-(aq) + Cd2+(aq) has a measured standard cell potential of +1.03 V. (c) Sketch the voltaic cell, label the anode and cathode, and indicate the direction of electron flow
 Verified step by step guidance
Verified step by step guidance1
Identify the oxidation and reduction half-reactions. In this reaction, Cd(s) is oxidized to Cd2+(aq), and PdCl4^2-(aq) is reduced to Pd(s).
Determine the anode and cathode. The anode is where oxidation occurs, so Cd(s) is the anode. The cathode is where reduction occurs, so PdCl4^2-(aq) is the cathode.
Sketch the voltaic cell. Draw two compartments: one for the anode and one for the cathode. Connect them with a salt bridge to allow the flow of ions.
Label the anode and cathode in your sketch. The anode (Cd) should be on the left, and the cathode (Pd) should be on the right.
Indicate the direction of electron flow. Electrons flow from the anode to the cathode through the external circuit. In this case, electrons flow from the Cd electrode to the Pd electrode.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Voltaic Cell Components
A voltaic cell, also known as a galvanic cell, consists of two electrodes: the anode and the cathode, immersed in an electrolyte solution. The anode is where oxidation occurs, releasing electrons, while the cathode is where reduction takes place, accepting electrons. Understanding these components is essential for sketching the cell and labeling its parts correctly.
Recommended video:
Guided course
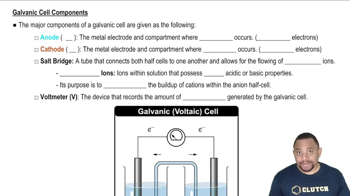
Galvanic Cell Components
Standard Cell Potential
The standard cell potential (E°) is a measure of the voltage produced by a voltaic cell under standard conditions (1 M concentration, 1 atm pressure, and 25°C). It indicates the driving force behind the electrochemical reaction, with a positive value suggesting a spontaneous reaction. In this case, the +1.03 V indicates that the reaction is favorable and helps determine the direction of electron flow.
Recommended video:
Guided course

Standard Cell Potential
Electron Flow Direction
In a voltaic cell, electrons flow from the anode to the cathode through an external circuit. This flow occurs because the anode is negatively charged (due to oxidation) and the cathode is positively charged (due to reduction). Understanding this flow is crucial for accurately sketching the cell and indicating the movement of electrons during the electrochemical reaction.
Recommended video:
Guided course

Electron Geometry
Related Practice
Textbook Question
1
views
Textbook Question
A voltaic cell that uses the reaction PdCl42-(aq) + Cd(s) → Pd(s) + 4 Cl-(aq) + Cd2+(aq) has a measured standard cell potential of +1.03 V. (b) By using data from Appendix E, determine E°red for the reaction involving Pd.
Textbook Question
Using standard reduction potentials (Appendix E), calculate the standard emf for each of the following reactions: (a) Cl21g2 + 2 I-1aq2 ¡ 2 Cl-1aq2 + I21s2
Textbook Question
Using standard reduction potentials (Appendix E), calculate the standard emf for each of the following reactions: (b) Ni1s2 + 2 Ce4+1aq2 ¡ Ni2+1aq2 + 2 Ce3+1aq2
Textbook Question
Using standard reduction potentials (Appendix E), calculate the standard emf for each of the following reactions: (c) Fe1s2 + 2 Fe3+1aq2 ¡ 3 Fe2+1aq2
