Textbook Question
Which of the following are ionic, and which are molecular? (a) PF5

 Verified step by step guidance
Verified step by step guidance

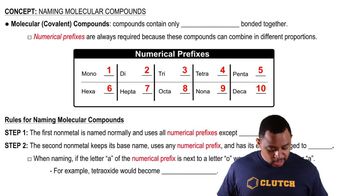

Which of the following are ionic, and which are molecular? (a) PF5
Which of the following are ionic, and which are molecular? (b) NaI (c) Zr(NO3)2
Which of the following are ionic, and which are molecular? (d) Ca(NO3)2
Which of the following are ionic, and which are molecular? (g) CoCO3
Which of the following are ionic, and which are molecular? (h) N2O4
Give the chemical formula for (a) chromate ion (b) bromide iom (c) nitrite ion (d) sulphite ion