
The radius of an atom of tungsten (W) is about 2.10 Å. (c) If the atom is assumed to be a sphere, what is the volume in m3 of a single W atom?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
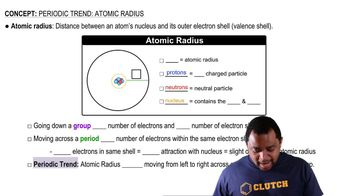
Atomic Radius
Volume of a Sphere
Unit Conversion

Millikan determined the charge on the electron by studying the static charges on oil drops falling in an electric field (Figure 2.5). A student carried out this experiment using several oil drops for her measurements and calculated the charges on the drops. She obtained the following data: Droplet Calculated Charge (C) A 1.60 * 10-19 B 3.15 * 10-19 C 4.81 * 10-19 D 6.31 * 10-19 (b) What conclusion can the student draw from these data regarding the charge of the electron?
Millikan determined the charge on the electron by studying the static charges on oil drops falling in an electric field (Figure 2.5). A student carried out this experiment using several oil drops for her measurements and calculated the charges on the drops. She obtained the following data: Droplet Calculated Charge (C) A 1.60 * 10-19 B 3.15 * 10-19 C 4.81 * 10-19 D 6.31 * 10-19 (c) What value (and to how many significant figures) should she report for the electronic charge?
The radius of an atom of copper (Cu) is about 140 pm. (a) Express this distance in angstroms 1A 2.
The radius of an atom of copper (Cu) is about 140 pm. (b) How many Cu atoms would have to be placed side by side to span a distance of 5.0 mm?
The radius of an atom of copper (Cu) is about 140 pm. (c) If you assume that the Cu atom is a sphere, what is the volume in cm3 of a single atom?
