Only two isotopes of copper occur naturally: 63Cu (atomic mass = 62.9296 amu; abundance 69.17%) 65Cu (atomic mass = 64.9278 amu; abundance 30.83%). Calculate the atomic weight (average atomic mass) of copper.
Ch.2 - Atoms, Molecules, and Ions

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 2, Problem 37a
(a) Thomson's cathode-ray tube (Figure 2.4) and the mass spectrometer (Figure 2.11) both involve the use of electric or magnetic fields to deflect charged particles. What are the charged particles involved in each of these experiments?
 Verified step by step guidance
Verified step by step guidance1
Identify the charged particles in Thomson's cathode-ray tube experiment. These are electrons, which are negatively charged particles.
Understand that in Thomson's experiment, the cathode rays are streams of electrons emitted from the cathode in a vacuum tube.
Recognize that in a mass spectrometer, the charged particles are ions. These ions can be positively or negatively charged, depending on the type of mass spectrometer and the sample being analyzed.
In a mass spectrometer, ions are generated from the sample and then accelerated through electric and magnetic fields, which separate them based on their mass-to-charge ratio.
Compare the two experiments: Thomson's cathode-ray tube focuses on the deflection of electrons, while the mass spectrometer focuses on the deflection and separation of ions based on their mass-to-charge ratio.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
7mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Cathode Rays
Cathode rays are streams of electrons emitted from the cathode in a vacuum tube. In Thomson's cathode-ray tube experiment, these negatively charged particles are deflected by electric and magnetic fields, allowing for the determination of their charge-to-mass ratio. This experiment was pivotal in the discovery of the electron as a fundamental particle.
Recommended video:
Guided course
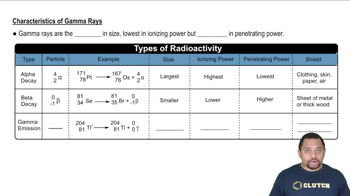
Characteristics of Gamma Rays
Mass Spectrometry
Mass spectrometry is an analytical technique used to measure the mass-to-charge ratio of ions. In a mass spectrometer, charged particles (ions) are generated and then subjected to electric and magnetic fields, which separate them based on their mass. This allows for the identification and quantification of different isotopes and molecules in a sample.
Recommended video:
Guided course

Atomic Mass
Electric and Magnetic Fields
Electric and magnetic fields are fundamental forces that can influence the motion of charged particles. In both Thomson's cathode-ray tube and mass spectrometry, these fields are used to manipulate the trajectory of electrons or ions, enabling scientists to study their properties. The deflection of these particles provides insights into their charge and mass, which are critical for understanding atomic structure.
Recommended video:
Guided course
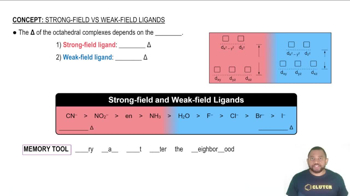
Strong-Field Ligands result in a large Δ and Weak-Field Ligands result in a small Δ.
Related Practice
Textbook Question
4
views
Textbook Question
Rubidium has two naturally occurring isotopes, rubidium-85 (atomic mass = 84.9118 amu; abundance = 72.15%) and rubidium-87 (atomic mass = 86.9092 amu; abundance = 27.85%). Calculate the atomic weight of rubidium
Textbook Question
Consider the mass spectrometer shown in Figure 2.11. Determine whether each of the following statements is true or false. If false, correct the statement to make it true: (a) The paths of neutral (uncharged) atoms are not affected by the magnet.
Textbook Question
Consider the mass spectrometer shown in Figure 2.11. Determine whether each of the following statements is true or false. If false, correct the statement to make it true: (b) The height of each peak in the mass spectrum is inversely proportional to the mass of that isotope.
1
views
