Sodium reacts with oxygen in air to form two compounds: sodium oxide and sodium peroxide. In forming sodium oxide, 23.0 g of sodium combines with 8.0 g of hydrogen. In forming sodium peroxide, 23.0 g of sodium combines with 16.0 g of oxygen. (a) What are the mass ratios of oxygen in the two compounds?

A 1.0-g sample of carbon dioxide (CO2) is fully decomposed into its elements, yielding 0.273 g of carbon and 0.727 g of oxygen. (b) If a sample of a different compound decomposes into 0.429 g of carbon and 0.571 g of oxygen, what is its ratio of the mass of O to C?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Law of Conservation of Mass
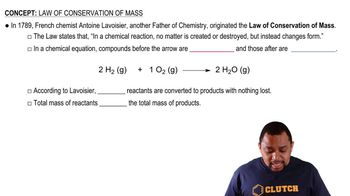
Mass Ratio
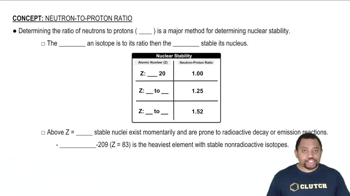
Stoichiometry

The following diagram represents an ionic compound in which the red spheres represent cations and the blue spheres represent anions. Which of the following formulas is consistent with the drawing? KBr, K2SO4, Ca1NO322, Fe21SO423.
In the Millikan oil-drop experiment (see Figure 2.5), the tiny oil drops are observed through the viewing lens as rising, stationary, or falling, as shown here. (a) What causes their rate of fall to vary from their rate in the absence of an electric field?
A 1.0-g sample of carbon dioxide (CO2) is fully decomposed into its elements, yielding 0.273 g of carbon and 0.727 g of oxygen. (a) What is the ratio of the mass of O to C?
A 1.0-g sample of carbon dioxide (CO2) is fully decomposed into its elements, yielding 0.273 g of carbon and 0.727 g of oxygen. If a sample of a different compound decomposes into 0.429 g of carbon and 0.571 g of oxygen, what is its ratio of the mass of O to C? (c) According to Dalton's atomic theory, what is the empirical formula of the second compound?
Sodium reacts with oxygen in air to form two compounds: sodium oxide and sodium peroxide. In forming sodium oxide, 23.0 g of sodium combines with 8.0 g of hydrogen. In forming sodium peroxide, 23.0 g of sodium combines with 16.0 g of oxygen. (b) What fundamental law does this experiment demonstrate?
