Textbook Question
The value of Ka for nitrous acid (HNO2) at 25 °C is given in Appendix D. (a) Write the chemical equation for the equilibrium that corresponds to Ka.

 Verified step by step guidance
Verified step by step guidance
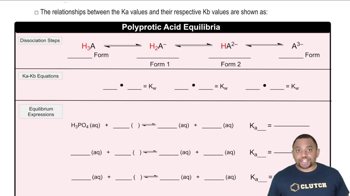


The value of Ka for nitrous acid (HNO2) at 25 °C is given in Appendix D. (a) Write the chemical equation for the equilibrium that corresponds to Ka.
The value of Ka for nitrous acid (HNO2) at 25 °C is given in Appendix D. (b) By using the value of Ka, calculate ΔG° for the dissociation of nitrous acid in aqueous solution.
The value of Ka for nitrous acid (HNO2) at 25 °C is given in Appendix D. (c) What is the value of ΔG at equilibrium?
The Kb for methylamine (CH3NH2) at 25 °C is given in Appendix D. (a) Write the chemical equation for the equilibrium that corresponds to Kb.
The Kb for methylamine (CH3NH2) at 25 °C is given in Appendix D. (d) What is the value of ΔG when [H+] = 6.7 × 10-9 M, [CH3NH3+] = 2.4 × 10-3 M, and [CH3NH2] = 0.098 M?