Textbook Question
(a) For a process that occurs at constant temperature, does the change in Gibbs free energy depend on changes in the enthalpy and entropy of the system?
1
views

 Verified step by step guidance
Verified step by step guidance
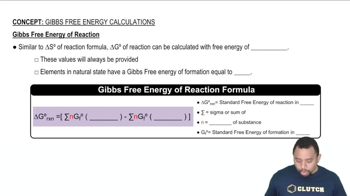
(a) For a process that occurs at constant temperature, does the change in Gibbs free energy depend on changes in the enthalpy and entropy of the system?
(b) For a certain process that occurs at constant T and P, the value of ΔG is positive. Is the process spontaneous?
For a certain chemical reaction, ΔH° = -35.4 kJ and ΔS° = -85.5 J/K. (b) Does the reaction lead to an increase or decrease in the randomness or disorder of the system?
For a certain chemical reaction, ΔH° = -35.4 kJ and ΔS° = -85.5 J/K. (c) Calculate ΔG° for the reaction at 298 K. (d) Is the reaction spontaneous at 298 K under standard conditions?