Which of the following processes are spontaneous and which are nonspontaneous: (e) formation of CH4 and O2 molecules from CO2 and H2O at room temperature and 1 atm of pressure?

(a) Can endothermic chemical reactions be spontaneous? (b) Can a process be spontaneous at one temperature and nonspontaneous at a different temperature? (c) Water can be decomposed to form hydrogen and oxygen, and the hydrogen and oxygen can be recombined to form water. Does this mean that the processes are thermodynamically reversible?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Spontaneity of Reactions

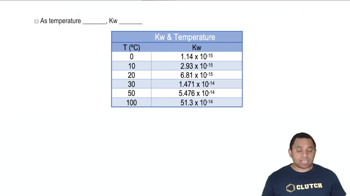
Temperature Dependence of Spontaneity
Thermodynamic Reversibility

Which of the following processes are spontaneous?
a. the melting of ice cubes at −10 °C and 1 atm pressure
b. separating a mixture of N2 and O2 into two separate samples, one that is pure N2 and one that is pure O2
c. alignment of iron filings in a magnetic field
d. the reaction of hydrogen gas with oxygen gas to form water vapor at room temperature
e. the dissolution of HCl(g) in water to form concentrated hydrochloric acid
Indicate whether each statement is true or false. (a) A reaction that is spontaneous in one direction will be nonspontaneous in the reverse direction under the same reaction conditions. (b) All spontaneous processes are fast. (c) Most spontaneous processes are reversible. (d) An isothermal process is one in which the system loses no heat. (e) The maximum amount of work can be accomplished by an irreversible process rather than a reversible one.
(d) Does the amount of work that a system can do on its surroundings depend on the path of the process?
Consider the vaporization of liquid water to steam at a pressure of 1 atm. (a) Is this process endothermic or exothermic?
Consider the vaporization of liquid water to steam at a pressure of 1 atm. (b) In what temperature range is it a spontaneous process?
