Textbook Question
A 1.00-L solution saturated at 25 C with calcium oxalate 1CaC2O42 contains 0.0061 g of CaC2O4. Calculate the solubility-product constant for this salt at 25 C.

 Verified step by step guidance
Verified step by step guidance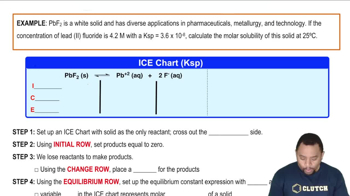


A 1.00-L solution saturated at 25 C with calcium oxalate 1CaC2O42 contains 0.0061 g of CaC2O4. Calculate the solubility-product constant for this salt at 25 C.
(b) If 0.0490 g of AgIO3 dissolves per liter of solution, calculate the solubility-product constant.
A 1.00-L solution saturated at 25 C with lead(II) iodide contains 0.54 g of PbI2. Calculate the solubility-product constant for this salt at 25 C.
Calculate the solubility of LaF3 in grams per liter in (a) pure water.
Calculate the solubility of LaF3 in grams per liter in (b) 0.010 M KF solution.
Calculate the solubility of LaF3 in grams per liter in (c) 0.050 M LaCl3 solution.