The beaker on the right contains 0.1 M acetic acid solution with methyl orange as an indicator. The beaker on the left contains a mixture of 0.1 M acetic acid and 0.1 M sodium acetate with methyl orange. (a) Using Figures 16.8 and 16.9, which solution has a higher pH?
Ch.17 - Additional Aspects of Aqueous Equilibria

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 17, Problem 1
Tooth enamel is composed of hydroxyapatite, whose simplest formula is Ca5(PO4)3OH, and whose corresponding Ksp = 6.8 × 10^(-27). As discussed in the Chemistry and Life box on page 746, fluoride in fluorinated water or in toothpaste reacts with hydroxyapatite to form fluoroapatite, Ca5(PO4)3F, whose Ksp = 1.0 × 10^(-60). (b) Calculate the molar solubility of each of these compounds.
 Verified step by step guidance
Verified step by step guidance1
Identify the dissolution reactions for both hydroxyapatite and fluoroapatite. For hydroxyapatite, the reaction is: Ca_5(PO_4)_3OH (s) ⇌ 5Ca^{2+} (aq) + 3PO_4^{3-} (aq) + OH^{-} (aq). For fluoroapatite, the reaction is: Ca_5(PO_4)_3F (s) ⇌ 5Ca^{2+} (aq) + 3PO_4^{3-} (aq) + F^{-} (aq).
Write the expression for the solubility product constant (K_{sp}) for each compound. For hydroxyapatite: K_{sp} = [Ca^{2+}]^5 [PO_4^{3-}]^3 [OH^{-}]. For fluoroapatite: K_{sp} = [Ca^{2+}]^5 [PO_4^{3-}]^3 [F^{-}].
Assume the molar solubility of hydroxyapatite is 's'. Then, [Ca^{2+}] = 5s, [PO_4^{3-}] = 3s, and [OH^{-}] = s. Substitute these into the K_{sp} expression for hydroxyapatite and solve for 's'.
Similarly, assume the molar solubility of fluoroapatite is 's'. Then, [Ca^{2+}] = 5s, [PO_4^{3-}] = 3s, and [F^{-}] = s. Substitute these into the K_{sp} expression for fluoroapatite and solve for 's'.
Compare the molar solubilities of hydroxyapatite and fluoroapatite to understand the effect of fluoride on the solubility of tooth enamel.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Solubility Product Constant (Ksp)
The solubility product constant (Ksp) is an equilibrium constant that quantifies the solubility of a sparingly soluble ionic compound. It is defined as the product of the molar concentrations of the ions, each raised to the power of their coefficients in the balanced equation. A lower Ksp value indicates a lower solubility in water, which is crucial for comparing the solubility of hydroxyapatite and fluoroapatite in this context.
Recommended video:
Guided course

Solubility Product Constant
Molar Solubility
Molar solubility refers to the number of moles of a solute that can dissolve in a liter of solution at equilibrium. It is directly related to Ksp, as the molar solubility can be calculated from the Ksp expression for a given compound. Understanding how to derive molar solubility from Ksp is essential for solving the problem regarding the solubility of hydroxyapatite and fluoroapatite.
Recommended video:
Guided course
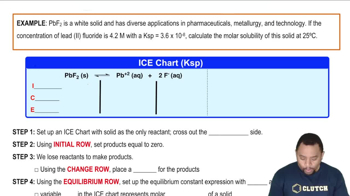
Molar Solubility Example
Equilibrium in Chemical Reactions
Equilibrium in chemical reactions occurs when the rates of the forward and reverse reactions are equal, resulting in constant concentrations of reactants and products. In the context of solubility, this means that the dissolution of a solid and the precipitation of its ions reach a balance. Recognizing how equilibrium affects the solubility of compounds like hydroxyapatite and fluoroapatite is vital for calculating their molar solubility.
Recommended video:
Guided course
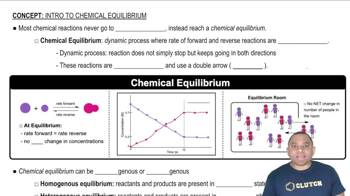
Chemical Equilibrium Concepts
Related Practice
Textbook Question
Textbook Question
The beaker on the right contains 0.1 M acetic acid solution with methyl orange as an indicator. The beaker on the left contains a mixture of 0.1 M acetic acid and 0.1 M sodium acetate with methyl orange. (b) Which solution is better able to maintain its pH when small amounts of NaOH are added? Explain. [Sections 17.1 and 17.2]
2
views
Textbook Question
A buffer contains a weak acid, HA, and its conjugate base. The weak acid has a pKa of 4.5, and the buffer has a pH of 4.3. Without doing a calculation, state which of these possibilities are correct at pH 4.3. (a) 3HA4 = 3A-4, (b) 3HA4 7 3A-4, or (c) 3HA4 6 3A-4. [Section 17.2]
1
views
