The solubility product for Zn1OH22 is 3.0 * 10-16. The formation constant for the hydroxo complex, Zn1OH242 - , is 4.6 * 1017. What concentration of OH- is required to dissolve 0.015 mol of Zn1OH22 in a liter of solution?
Ch.17 - Additional Aspects of Aqueous Equilibria

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 17, Problem 109a
The value of Ksp for Cd(OH)2 is 2.5 × 10-14. (a) What is the molar solubility of Cd(OH)2?
 Verified step by step guidance
Verified step by step guidance1
Identify the dissolution equation for Cd(OH)2: Cd(OH)2(s) ⇌ Cd2+(aq) + 2OH-(aq).
Write the expression for the solubility product constant (Ksp): Ksp = [Cd2+][OH-]2.
Let the molar solubility of Cd(OH)2 be 's'. Then, [Cd2+] = s and [OH-] = 2s.
Substitute the expressions for the ion concentrations into the Ksp expression: Ksp = (s)(2s)2 = 4s3.
Set the Ksp expression equal to the given Ksp value and solve for 's': 4s3 = 2.5 × 10-14.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
5mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Solubility Product Constant (Ksp)
The solubility product constant (Ksp) is an equilibrium constant that applies to the solubility of sparingly soluble ionic compounds. It is defined as the product of the molar concentrations of the ions, each raised to the power of their coefficients in the balanced equation. For Cd(OH)2, Ksp = [Cd^2+][OH^-]^2, which helps determine how much of the compound can dissolve in water.
Recommended video:
Guided course

Solubility Product Constant
Molar Solubility
Molar solubility refers to the maximum concentration of a solute that can dissolve in a given volume of solvent at equilibrium. It is typically expressed in moles per liter (mol/L). In the case of Cd(OH)2, calculating the molar solubility involves determining the concentration of Cd^2+ and OH^- ions that can exist in solution when the compound is at its saturation point.
Recommended video:
Guided course
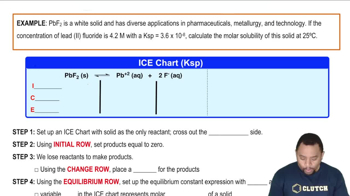
Molar Solubility Example
Stoichiometry of Dissolution
The stoichiometry of dissolution describes the ratio in which ions are produced when a compound dissolves. For Cd(OH)2, the dissolution can be represented as Cd(OH)2(s) ⇌ Cd^2+(aq) + 2OH^-(aq). This indicates that one mole of Cd(OH)2 produces one mole of Cd^2+ and two moles of OH^-, which is essential for relating Ksp to molar solubility.
Recommended video:
Guided course

Stoichiometry Concept
Related Practice
Textbook Question
Textbook Question
The value of Ksp for Cd(OH)2 is 2.5 × 10–14. (b) The solubility of Cd(OH)2 can be increased through formation of the complex ion CdBr42- (Kf = 5 × 103). If solid Cd(OH)2 is added to a NaBr solution, what is the initial concentration of NaBr needed to increase the molar solubility of Cd(OH)2 to 1.0 × 10-3 mol/L?
Textbook Question
(a) Write the net ionic equation for the reaction that occurs when a solution of hydrochloric acid (HCl) is mixed with a solution of sodium formate 1NaCHO22.
Textbook Question
(a) A 0.1044-g sample of an unknown monoprotic acid requires 22.10 mL of 0.0500 M NaOH to reach the end point. What is the molar mass of the unknown?
