Consider the titration of 30.0 mL of 0.050 M NH3 with 0.025 M HCl. Calculate the pH after the following volumes of titrant have been added: (e) 61.0 mL (f) 65.0 mL.
Ch.17 - Additional Aspects of Aqueous Equilibria

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 17, Problem 48a
Calculate the pH at the equivalence point in titrating 0.100 M solutions of each of the following with 0.080 M NaOH: (a) hydrobromic acid (HBr).
 Verified step by step guidance
Verified step by step guidance1
Identify the type of acid involved in the titration. Hydrobromic acid (HBr) is a strong acid.
Recognize that at the equivalence point, the moles of acid equal the moles of base added. Since HBr is a strong acid, it will completely dissociate in water.
Write the balanced chemical equation for the reaction: \[ \text{HBr} + \text{NaOH} \rightarrow \text{NaBr} + \text{H}_2\text{O} \]
At the equivalence point, the solution contains the salt NaBr. Since NaBr is formed from a strong acid and a strong base, it does not affect the pH of the solution.
Conclude that the pH at the equivalence point for the titration of a strong acid with a strong base is neutral, which is a pH of 7.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Titration and Equivalence Point
Titration is a quantitative analytical method used to determine the concentration of a solute in a solution. The equivalence point occurs when the amount of titrant added is stoichiometrically equivalent to the amount of analyte in the solution, resulting in complete neutralization. At this point, the pH of the solution is determined by the properties of the resulting solution, which can vary depending on the strength of the acid and base involved.
Recommended video:
Guided course
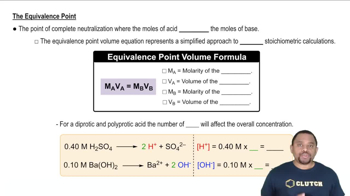
Equivalence Point in Titration
Strong Acid vs. Strong Base
Hydrobromic acid (HBr) is a strong acid that completely dissociates in solution, while sodium hydroxide (NaOH) is a strong base that also fully dissociates. In a titration involving a strong acid and a strong base, the pH at the equivalence point is typically neutral (pH 7) because the resulting solution contains only water and the salt formed from the acid and base, which does not affect the pH significantly.
Recommended video:
Guided course

Strong Acid-Strong Base Titration
pH Calculation
The pH is a measure of the hydrogen ion concentration in a solution, calculated using the formula pH = -log[H+]. At the equivalence point of a strong acid-strong base titration, the pH can be calculated based on the concentrations of the reactants and the volume of titrant added. Since both HBr and NaOH are strong, the resulting solution at the equivalence point will have a pH close to 7, indicating neutrality.
Recommended video:
Guided course

pH Calculation Example
Related Practice
Textbook Question
Textbook Question
Calculate the pH at the equivalence point for titrating 0.200 M solutions of each of the following bases with 0.200 M HBr: (a) sodium hydroxide (NaOH).
Textbook Question
Calculate the pH at the equivalence point for titrating 0.200 M solutions of each of the following bases with 0.200 M HBr: (b) hydroxylamine 1NH2OH2.
Textbook Question
Calculate the pH at the equivalence point in titrating 0.100 M solutions of each of the following with 0.080 M NaOH: (b) chlorous acid (HClO2).
Textbook Question
Calculate the pH at the equivalence point in titrating 0.100 M solutions of each of the following with 0.080 M NaOH: (c) benzoic acid (C6H5COOH).
