A 1.00-L solution saturated at 25 C with calcium oxalate 1CaC2O42 contains 0.0061 g of CaC2O4. Calculate the solubility-product constant for this salt at 25 C.
Ch.17 - Additional Aspects of Aqueous Equilibria

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 17, Problem 54
(c) Using the appropriate Ksp value from Appendix D, calculate the pH of a saturated solution of Ca(OH)2.
 Verified step by step guidance
Verified step by step guidance1
Identify the dissolution reaction for calcium hydroxide: Ca(OH)2(s) ⇌ Ca2+(aq) + 2OH-(aq).
Write the expression for the solubility product constant (Ksp) for Ca(OH)2: Ksp = [Ca2+][OH-]2.
Let 's' be the solubility of Ca(OH)2 in mol/L. Then, [Ca2+] = s and [OH-] = 2s.
Substitute the expressions for [Ca2+] and [OH-] into the Ksp expression: Ksp = (s)(2s)2 = 4s3.
Solve for 's' using the given Ksp value, then calculate [OH-] = 2s. Use the relation pOH = -log[OH-] and pH + pOH = 14 to find the pH of the solution.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Solubility Product Constant (Ksp)
The solubility product constant (Ksp) is an equilibrium constant that applies to the solubility of sparingly soluble ionic compounds. It quantifies the extent to which a compound can dissolve in water, represented by the concentrations of its ions at equilibrium. For calcium hydroxide, Ca(OH)2, the Ksp expression is derived from the dissociation of the compound into calcium ions and hydroxide ions.
Recommended video:
Guided course

Solubility Product Constant
pH and Hydroxide Ion Concentration
pH is a measure of the acidity or basicity of a solution, defined as the negative logarithm of the hydrogen ion concentration. In the case of a saturated solution of Ca(OH)2, the concentration of hydroxide ions (OH-) produced will influence the pH. Since Ca(OH)2 is a strong base, the higher the concentration of OH- ions, the higher the pH, indicating a more basic solution.
Recommended video:
Guided course
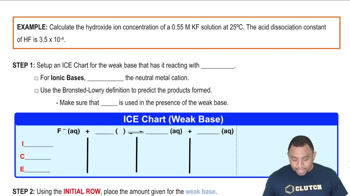
Hydroxide Ion Concentration Example
Equilibrium Calculations
Equilibrium calculations involve determining the concentrations of reactants and products at equilibrium in a chemical reaction. For the dissolution of Ca(OH)2, one must set up an equilibrium expression using the Ksp value and the concentrations of Ca2+ and OH- ions. This allows for the calculation of the hydroxide ion concentration, which can then be used to find the pH of the solution.
Recommended video:
Guided course

Equilibrium Constant Calculation
Related Practice
Textbook Question
Textbook Question
(b) Write the expression for the solubility-product constant for each of the following ionic compounds: MnCO3, Hg(OH)2, and Cu3(PO4)3.
Textbook Question
(a) I f t he molar solubility of CaF2 at 35°C i s 1.24 × 10–3 mol/L, what is Ksp at this temperature?
Textbook Question
(b) It is found that 1.1 × 10-2 g SrF2 dissolves per 100 mL of aqueous solution at 25°C. Calculate the solubility product for SrF2.
Textbook Question
(b) If 0.0490 g of AgIO3 dissolves per liter of solution, calculate the solubility-product constant.
Textbook Question
A 1.00-L solution saturated at 25 C with lead(II) iodide contains 0.54 g of PbI2. Calculate the solubility-product constant for this salt at 25 C.
