Dilution and Concentration
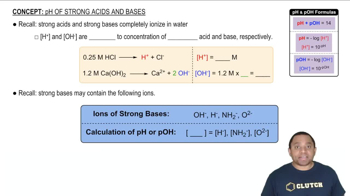
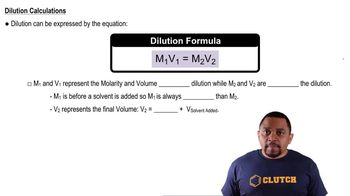
When a solution is diluted, the number of moles of solute remains constant, but the volume increases, resulting in a lower concentration. The dilution can be calculated using the formula C1V1 = C2V2, where C1 and V1 are the initial concentration and volume, and C2 and V2 are the final concentration and volume. This concept is crucial for determining the pH of diluted strong acid solutions.

 Verified step by step guidance
Verified step by step guidance