Indicate whether each of the following statements is correct or incorrect. (c) Conjugate acids of weak bases produce more acidic solutions than conjugate acids of strong bases.
Ch.16 - Acid-Base Equilibria

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 16, Problem 100
A solution is made by adding 0.300 g Ca1OH221s2, 50.0 mL of 1.40 M HNO3, and enough water to make a final volume of 75.0 mL. Assuming that all of the solid dissolves, what is the pH of the final solution?
 Verified step by step guidance
Verified step by step guidance1
Calculate the moles of Ca(OH)_2 using its molar mass.
Determine the moles of HNO_3 using its concentration and volume.
Write the balanced chemical equation for the reaction: Ca(OH)_2 + 2HNO_3 -> Ca(NO_3)_2 + 2H_2O.
Calculate the moles of H^+ ions remaining after the reaction, considering the stoichiometry of the reaction.
Use the concentration of remaining H^+ ions in the final solution volume to find the pH using the formula: pH = -log[H^+].

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
6mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Dissociation of Calcium Hydroxide
Calcium hydroxide, Ca(OH)2, is a strong base that dissociates completely in water to produce calcium ions (Ca²⁺) and hydroxide ions (OH⁻). The dissociation reaction can be represented as Ca(OH)2 → Ca²⁺ + 2OH⁻. Understanding this dissociation is crucial for determining the concentration of hydroxide ions in the solution, which directly affects the pH.
Recommended video:
Guided course
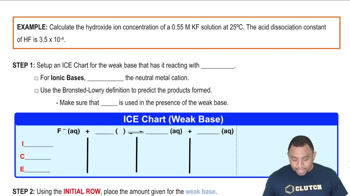
Hydroxide Ion Concentration Example
Strong Acid-Base Neutralization
In this scenario, nitric acid (HNO3) is a strong acid that also dissociates completely in solution to yield hydrogen ions (H⁺). The reaction between the hydroxide ions from calcium hydroxide and the hydrogen ions from nitric acid leads to neutralization, forming water. The stoichiometry of this reaction is essential for calculating the resulting concentrations of H⁺ and OH⁻ ions, which ultimately determines the pH of the solution.
Recommended video:
Guided course

Strong Acid-Strong Base Titration
pH Calculation
pH is a measure of the acidity or basicity of a solution, defined as the negative logarithm of the hydrogen ion concentration: pH = -log[H⁺]. In a neutralization reaction, the final pH depends on the relative amounts of H⁺ and OH⁻ ions present after the reaction. If there is an excess of H⁺, the solution will be acidic (pH < 7), while an excess of OH⁻ will result in a basic solution (pH > 7).
Recommended video:
Guided course

pH Calculation Example
Related Practice
Textbook Question
1
views
Textbook Question
Indicate whether each of the following statements is correct or incorrect. (d) K+ ion is acidic in water because it causes hydrating water molecules to become more acidic.
Textbook Question
Salts containing the phosphate ion are added to municipal water supplies to prevent the corrosion of lead pipes. (a) Based on the pKa values for phosphoric acid 1pKa1 = 7.5 * 10 - 3, pKa2 = 6.2 * 10 - 8, pKa3 = 4.2 * 10 - 132 what is the Kb value for the PO43 - ion?
Textbook Question
Which, if any, of the following statements are true? (a) The stronger the base, the smaller the pKb. (b) The stronger the base, the larger the pKb. (c) The stronger the base, the smaller the Kb. (d) The stronger the base, the larger the Kb. (e) The stronger the base, the smaller the pKa of its conjugate acid. (f) The stronger the base, the larger the pKa of its conjugate acid.
Textbook Question
Predict how each molecule or ion would act, in the Brønsted-Lowry sense, in aqueous solution by writing 'acid,' 'base,' 'both,' or 'neither' on the line provided. (b) Prozac
