When lead(IV) oxide is heated above 300°C, it decomposes according to the reaction, 2 PbO2(𝑠) ⇌ 2PbO(𝑠) + O2(𝑔). Consider the two sealed vessels of PbO2 shown here. If both vessels are heated to 400°C and allowed to come to equilibrium, which of the following statements is or are true? a. There will be less PbO2 remaining in vessel A,
Ch.15 - Chemical Equilibrium

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 15, Problem 7c
When lead(IV) oxide is heated above 300°C, it decomposes according to the reaction, 2 PbO2(𝑠) ⇌ 2PbO(𝑠) + O2(𝑔). Consider the two sealed vessels of PbO2 shown here. If both vessels are heated to 400°C and allowed to come to equilibrium, which of the following statements is or are true? (c) The amount of PbO2 remaining in each vessel will be the same. [Find more in Section 15.4]
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the chemical reaction. The decomposition of lead(IV) oxide (PbO_2) into lead(II) oxide (PbO) and oxygen gas (O_2) is a reversible reaction, represented as: 2 PbO_2(s) ⇌ 2 PbO(s) + O_2(g).
Step 2: Recognize the conditions for equilibrium. At equilibrium, the rate of the forward reaction (decomposition of PbO_2) equals the rate of the reverse reaction (formation of PbO_2 from PbO and O_2).
Step 3: Consider the effect of temperature. Both vessels are heated to 400°C, which is above the decomposition temperature of PbO_2, allowing the reaction to proceed until equilibrium is reached.
Step 4: Analyze the equilibrium position. The equilibrium position is determined by the equilibrium constant (K) at 400°C, which depends on the concentrations of the products and reactants, not the initial amounts of PbO_2.
Step 5: Evaluate the statement. Since the equilibrium position is the same for both vessels at the same temperature, the amount of PbO_2 remaining in each vessel at equilibrium will be the same, regardless of the initial amount of PbO_2.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
57sWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Chemical Equilibrium
Chemical equilibrium occurs when the rates of the forward and reverse reactions are equal, resulting in constant concentrations of reactants and products. In the context of the given reaction, this means that at 400°C, the amounts of PbO2, PbO, and O2 will stabilize at specific values, depending on the conditions and concentrations in the sealed vessels.
Recommended video:
Guided course

Chemical Equilibrium Concepts
Le Chatelier's Principle
Le Chatelier's Principle states that if a system at equilibrium is subjected to a change in concentration, temperature, or pressure, the system will adjust to counteract that change and restore a new equilibrium. In this case, heating the PbO2 will shift the equilibrium position, affecting the amounts of PbO2, PbO, and O2 present in the vessels.
Recommended video:
Guided course

Le Chatelier's Principle
Decomposition Reactions
Decomposition reactions involve a single compound breaking down into two or more products. The reaction of lead(IV) oxide decomposing into lead(II) oxide and oxygen gas is an example of this type of reaction. Understanding the nature of decomposition helps predict how the amounts of reactants and products will change as the system reaches equilibrium.
Recommended video:
Guided course
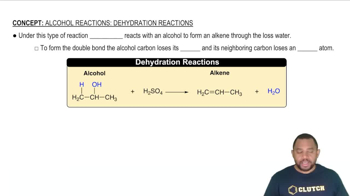
Alcohol Reactions: Dehydration Reactions
Related Practice
Textbook Question
Textbook Question
When lead(IV) oxide is heated above 300°C, it decomposes according to the reaction, 2 PbO2(𝑠)⇌2PbO(𝑠)+O2(𝑔). Consider the two sealed vessels of PbO2 shown here. If both vessels are heated to 400°C and allowed to come to equilibrium, which of the following statements is or are true?
b. There will be less PbO2 remaining in vessel B,
Textbook Question
The reaction A2 + B2 ⇌ 2 AB has an equilibrium constant Kc = 1.5. The following diagrams represent reaction mixtures containing A2 molecules (red), B2 molecules (blue), and AB molecules. (a) Which reaction mixture is at equilibrium?
1
views
Textbook Question
The diagram shown here represents the equilibrium state for the reaction A2(𝑔) + 2B(𝑔) ⇌ 2AB(𝑔). (a) Assuming the volume is 2 L, calculate the equilibrium constant 𝐾𝑐 for the reaction.
