Methanol (CH3OH) can be made by the reaction of CO with H2: CO(𝑔) + 2 H2(𝑔) ⇌ CH3OH(𝑔) (b) To maximize the equilibrium yield of methanol, would you use a high or low temperature?
Ch.15 - Chemical Equilibrium

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 15, Problem 66c
Methanol (CH3OH) can be made by the reaction of CO with H2: CO(𝑔) + 2 H2(𝑔) ⇌ CH3OH(𝑔) (c) To maximize the equilibrium yield of methanol, would you use a high or low pressure?
 Verified step by step guidance
Verified step by step guidance1
Understand the concept of Le Chatelier's Principle, which states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium moves to counteract the change.
Identify the number of moles of gas on both sides of the reaction: 1 mole of CO and 2 moles of H2 on the left (total 3 moles) and 1 mole of CH3OH on the right.
Recognize that increasing the pressure of a system in equilibrium favors the side of the reaction with fewer moles of gas. In this reaction, the right side with CH3OH has fewer moles of gas compared to the left side.
Conclude that to maximize the equilibrium yield of methanol, a high pressure should be used because it will shift the equilibrium towards the formation of methanol, which has fewer moles of gas.
Consider other factors that might affect the yield, such as temperature and catalysts, but for pressure specifically, a high pressure is favorable for increasing the yield of methanol in this reaction.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Le Chatelier's Principle
Le Chatelier's Principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to counteract the change. In the context of gas reactions, increasing pressure favors the side of the reaction with fewer moles of gas, while decreasing pressure favors the side with more moles.
Recommended video:
Guided course

Le Chatelier's Principle
Mole Ratio in Reactions
In the given reaction, the mole ratio of reactants to products is crucial for understanding how pressure affects equilibrium. The reaction CO(g) + 2 H2(g) ⇌ CH3OH(g) has three moles of gas on the reactant side and one mole on the product side, indicating that increasing pressure will favor the formation of methanol.
Recommended video:
Guided course
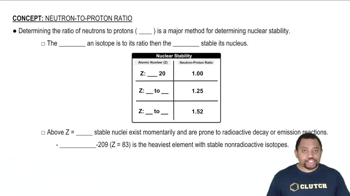
Neutron-Proton Ratio
Equilibrium Constant (K)
The equilibrium constant (K) quantifies the ratio of concentrations of products to reactants at equilibrium. Changes in pressure can influence the concentrations of gaseous reactants and products, thereby affecting the position of equilibrium and the yield of methanol. A higher pressure shifts the equilibrium towards the side with fewer gas moles, potentially increasing K for the product.
Recommended video:
Guided course

Equilibrium Constant K
Related Practice
Textbook Question
Textbook Question
Consider the following equilibrium between oxides of nitrogen
3 NO(g) ⇌ NO2(g) + N2O(g)
(c) At constant temperature, would a change in the volume of the container affect the fraction of products in the equilibrium mixture?
Textbook Question
Methanol (CH3OH) can be made by the reaction of CO with H2: CO(𝑔) + 2 H2(𝑔) ⇌ CH3OH(𝑔) (a) Use thermochemical data in Appendix C to calculate ΔH° for this reaction.
Textbook Question
Ozone, O3, decomposes to molecular oxygen in the stratosphere according to the reaction 2 O31g2¡3 O21g2. Would an increase in pressure favor the formation of ozone or of oxygen?
Textbook Question
The water–gas shift reaction CO1g2 + H2O1g2ΔCO21g2 + H21g2 is used industrially to produce hydrogen.The reaction enthalpy is H = -41 kJ.(b) Could you increase the equilibrium yieldof hydrogen by controlling the pressure of this reaction? Ifso would high or low pressure favor formation of H2(g)?
Textbook Question
(a) Is the dissociation of fluorine molecules into atomic fluorine, F2(𝑔) ⇌ 2 F(𝑔), an exothermic or endothermic process?
