For the equilibrium Br2(𝑔) + Cl2(𝑔) ⇌ 2 BrCl(𝑔) at 400 K, 𝐾𝑐 = 7.0. If 0.25 mol of Br2 and 0.55 mol of Cl2 are introduced into a 3.0-L container at 400 K, what will be the equilibrium concentrations of Br2, Cl2, and BrCl?

Consider the reaction \( \text{CaSO}_4(\text{s}) \rightleftharpoons \text{Ca}^{2+}(\text{aq}) + \text{SO}_4^{2-}(\text{aq}) \) At 25 °C, the equilibrium constant is \( K_c = 2.4 \times 10^{-5} \) for this reaction. (a) If excess \( \text{CaSO}_4(\text{s}) \) is mixed with water at 25 °C to produce a saturated solution of \( \text{CaSO}_4 \), what are the equilibrium concentrations of \( \text{Ca}^{2+} \) and \( \text{SO}_4^{2-} \)? (b) If the resulting solution has a volume of 1.4 L, what is the minimum mass of \( \text{CaSO}_4(\text{s}) \) needed to achieve equilibrium?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Equilibrium Constant (Kc)

Saturated Solution

Stoichiometry and Mass Calculations
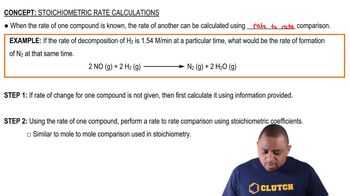
At 373 K, 𝐾𝑝 = 0.416 for the equilibrium 2 NOBr(𝑔) ⇌ 2 NO(𝑔) + Br2(𝑔) If the pressures of NOBr(𝑔) and NO(𝑔) are equal, what is the equilibrium pressure of Br2(𝑔)?
At 218°C, 𝐾𝑐 = 1.2×10−4 for the equilibrium NH4SH(𝑠) ⇌ NH3(𝑔) + H2S(𝑔) Calculate the equilibrium concentrations of NH3 and H2S if a sample of solid NH4SH is placed in a closed vessel at 218°C and decomposes until equilibrium is reached.
At 80°C, 𝐾𝑐 = 1.87×10−3 for the reaction PH3BCl3(𝑠) ⇌ PH3(𝑔) + BCl3(𝑔) (a) Calculate the equilibrium concentrations of PH3 and BCl3 if a solid sample of PH3BCl3 is placed in a closed vessel at 80°C and decomposes until equilibrium is reached.
At 80°C, 𝐾𝑐 = 1.87×10−3 for the reaction PH3BCl3(𝑠) ⇌ PH3(𝑔) + BCl3(𝑔) (a) Calculate the equilibrium concentrations of PH3 and BCl3 if a solid sample of PH3BCl3 is placed in a closed vessel at 80°C and decomposes until equilibrium is reached. (b) If the flask has a volume of 0.250 L, what is the minimum mass of PH3BCl3(𝑠) that must be added to the flask to achieve equilibrium?
