A sample of nitrosyl bromide (NOBr) decomposes according to the equation 2 NOBr(𝑔) ⇌ 2 NO(𝑔) + Br2(𝑔) An equilibrium mixture in a 5.00-L vessel at 100°C contains 3.22 g of NOBr, 3.08 g of NO, and 4.19 g of Br2. (b) What is the total pressure exerted by the mixture of gases?
Ch.15 - Chemical Equilibrium

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 15, Problem 77
Consider the hypothetical reaction A1g2 Δ 2 B1g2. A flask is charged with 0.75 atm of pure A, after which it is allowed to reach equilibrium at 0 _x001F_C. At equilibrium, the partial pressure of A is 0.36 atm. (a) What is the total pressure in the flask at equilibrium?
 Verified step by step guidance
Verified step by step guidance1
Start by writing the balanced chemical equation for the reaction: \( A(g) \rightarrow 2B(g) \). This indicates that one mole of A produces two moles of B.
Determine the change in pressure for A. Initially, the pressure of A is 0.75 atm, and at equilibrium, it is 0.36 atm. The change in pressure for A is \( 0.75 - 0.36 = 0.39 \) atm.
Use the stoichiometry of the reaction to find the change in pressure for B. Since 1 mole of A produces 2 moles of B, the change in pressure for B is \( 2 \times 0.39 = 0.78 \) atm.
Calculate the equilibrium pressure of B. Since B is formed from A, its equilibrium pressure is simply the change in pressure, which is 0.78 atm.
Add the equilibrium pressures of A and B to find the total pressure in the flask at equilibrium: \( 0.36 \text{ atm (A)} + 0.78 \text{ atm (B)} = 1.14 \text{ atm} \).
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Partial Pressure
Partial pressure is the pressure exerted by a single component of a gas mixture. In a system at equilibrium, the total pressure is the sum of the partial pressures of all gases present. Understanding how to calculate partial pressures is essential for determining the total pressure in a reaction involving gases.
Recommended video:
Guided course
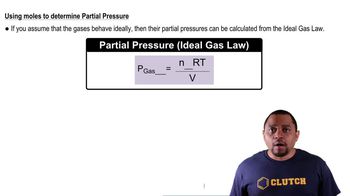
Partial Pressure Calculation
Equilibrium Constant
The equilibrium constant (K) is a value that expresses the ratio of the concentrations of products to reactants at equilibrium for a given reaction at a specific temperature. It helps predict the direction of the reaction and the concentrations of species at equilibrium. In this case, knowing the equilibrium constant can aid in understanding how the pressures of A and B relate to each other.
Recommended video:
Guided course

Equilibrium Constant K
Gas Laws
Gas laws describe the behavior of gases in relation to pressure, volume, and temperature. The ideal gas law (PV=nRT) is particularly relevant here, as it relates the total pressure, volume, and temperature of a gas mixture. Understanding these laws is crucial for calculating the total pressure in the flask after the reaction reaches equilibrium.
Recommended video:
Guided course
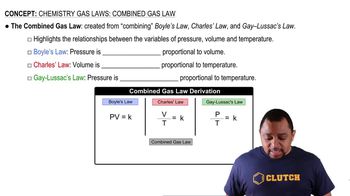
Combined Gas Law
Related Practice
Textbook Question
Textbook Question
A sample of nitrosyl bromide (NOBr) decomposes according to the equation 2 NOBr(g) ⇌ 2 NO(g) + Br2(g) An equilibrium mixture in a 5.00-L vessel at 100°C contains 3.22 g of NOBr, 3.08 g of NO, and 4.19 g of Br2. (c) What was the mass of the original sample of NOBr?
Textbook Question
Consider the hypothetical reaction A(g) ⇌ 2 B(g). A flask is charged with 0.75 atm of pure A, after which it is allowed to reach equilibrium at 0°C. At equilibrium, the partial pressure of A is 0.36 atm. (c) What could we do to maximize the yield of B?
