Textbook Question
Indicate whether each statement is true or false. (c) If you double the temperature for a reaction, you cut the activation energy in half.
1
views

 Verified step by step guidance
Verified step by step guidance
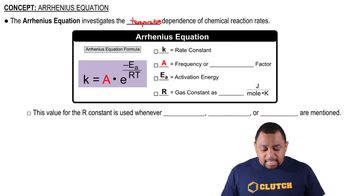

Indicate whether each statement is true or false. (c) If you double the temperature for a reaction, you cut the activation energy in half.
Based on their activation energies and energy changes and assuming that all collision factors are the same, rank the following reactions from slowest to fastest. (a) Ea = 45 kJ>mol; E = -25 kJ>mol (b) Ea = 35 kJ>mol; E = -10 kJ>mol (c) Ea = 55 kJ>mol; E = 10 kJ>mol
The temperature dependence of the rate constant for a reaction is tabulated as follows: Temperature (K) k 1M 1 s1 2 600 0.028 650 0.22 700 1.3 750 6.0 800 23 Calculate Ea and A.