As described in Exercise 14.41, the decomposition of sulfuryl chloride (SO2Cl2) is a first-order process. The rate constant for the decomposition at 660 K is 4.5 × 10-2 s-1. (b) At what time will the partial pressure of SO2Cl2 decline to one-tenth its initial value?
Ch.14 - Chemical Kinetics

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 14, Problem 44c
The first-order rate constant for the decomposition of N2O5, 2 N2O5(g) → 4 NO2(g) + O2(g), at 70°C is 6.82×10-3 s-1. Suppose we start with 0.0250 mol of N2O5(g) in a volume of 2.0 L. (c) What is the half-life of N2O5 at 70°C?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand that the half-life of a first-order reaction is given by the formula t1/2 = 0.693/k, where k is the rate constant.
Step 2: Identify the given rate constant (k) from the problem, which is 6.82 * 10^-3 s^-1.
Step 3: Substitute the given rate constant into the half-life formula.
Step 4: Solve the equation for t1/2 to find the half-life of N2O5 at 70 C.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
First-Order Reactions
First-order reactions are chemical reactions where the rate is directly proportional to the concentration of one reactant. In these reactions, the rate constant (k) remains constant, and the concentration of the reactant decreases exponentially over time. The half-life of a first-order reaction is independent of the initial concentration, making it a key characteristic for calculations involving such reactions.
Recommended video:
Guided course
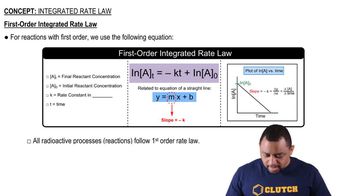
First-Order Reactions
Half-Life
The half-life of a substance is the time required for half of the initial amount of that substance to undergo a reaction or decay. For first-order reactions, the half-life can be calculated using the formula t1/2 = 0.693/k, where k is the rate constant. This concept is crucial for understanding how quickly a reactant is consumed in a reaction and is particularly useful in kinetics.
Recommended video:
Guided course
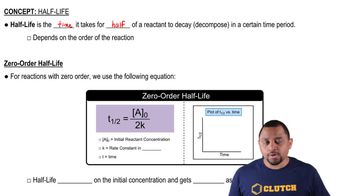
Zero-Order Half-life
Rate Constant
The rate constant (k) is a proportionality factor in the rate equation that relates the rate of a reaction to the concentration of reactants. It is specific to a given reaction at a particular temperature and is essential for calculating reaction rates and half-lives. In the context of first-order reactions, the rate constant directly influences the duration of the half-life and the speed of the reaction.
Recommended video:
Guided course
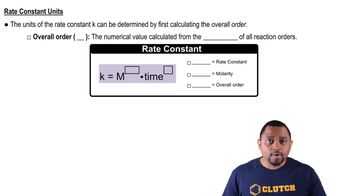
Rate Constant Units
Related Practice
Textbook Question
Textbook Question
The first-order rate constant for the decomposition of N2O5, 2 N2O5(g) → 4 NO2(g) + O2(g), at 70°C is 6.82×10-3 s-1. Suppose we start with 0.0250 mol of N2O5(g) in a volume of 2.0 L. (a) How many moles of N2O5 will remain after 5.0 min?
1
views
Textbook Question
From the following data for the first-order gas-phase isomerization of CH3NC at 215 C, calculate the firstorder rate constant and half-life for the reaction: Time (s) Pressure CH3nC (torr) 0 502 2000 335 5000 180 8000 95.5 12,000 41.7 15,000 22.4
Textbook Question
Consider the data presented in Exercise 14.19. (a) By using appropriate graphs, determine whether the reaction is first order or second order.
