The gas-phase reaction Cl(g) + HBr(g) → HCl(g) + Br(g) has an overall energy change of -66 kJ. The activation energy for the reaction is 7 kJ. (a) Sketch the energy profile for the reaction, and label Ea and ΔE.
Ch.14 - Chemical Kinetics

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 14, Problem 57
Indicate whether each statement is true or false. (a) If you compare two reactions with similar collision factors, the one with the larger activation energy will be slower. (b) A reaction that has a small rate constant must have a small frequency factor.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the concept of activation energy. Activation energy is the minimum energy required for a chemical reaction to occur. It is a barrier that reactants must overcome to transform into products.
Step 2: Analyze statement (a). The rate of a chemical reaction is influenced by the activation energy. According to the Arrhenius equation, a higher activation energy generally results in a slower reaction rate, assuming similar collision factors. This is because fewer molecules will have sufficient energy to overcome the higher barrier.
Step 3: Understand the Arrhenius equation: \( k = A e^{-\frac{E_a}{RT}} \), where \( k \) is the rate constant, \( A \) is the frequency factor, \( E_a \) is the activation energy, \( R \) is the gas constant, and \( T \) is the temperature in Kelvin.
Step 4: Analyze statement (b). The rate constant \( k \) is influenced by both the activation energy \( E_a \) and the frequency factor \( A \). A small rate constant does not necessarily imply a small frequency factor; it could also be due to a high activation energy.
Step 5: Consider the implications of the Arrhenius equation for both statements. For statement (a), a larger activation energy typically results in a slower reaction rate. For statement (b), a small rate constant can result from either a small frequency factor or a large activation energy.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Activation Energy
Activation energy is the minimum energy required for a chemical reaction to occur. It represents the energy barrier that reactants must overcome to transform into products. Reactions with higher activation energies typically proceed more slowly because fewer molecules have sufficient energy to surpass this barrier, leading to fewer successful collisions over time.
Recommended video:
Guided course

Activity Series Chart
Collision Theory
Collision theory posits that for a reaction to occur, reactant molecules must collide with sufficient energy and proper orientation. The frequency of these collisions, along with their energy, influences the reaction rate. If two reactions have similar collision factors but different activation energies, the one with the higher activation energy will have fewer effective collisions, resulting in a slower reaction rate.
Recommended video:
Guided course
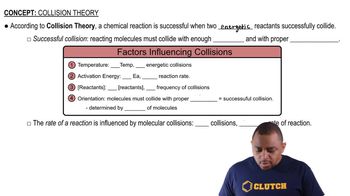
Collision Theory
Rate Constant and Frequency Factor
The rate constant (k) in a reaction is influenced by both the activation energy and the frequency factor (A), which reflects the frequency of collisions and the orientation of reactants. A small rate constant can indicate a slow reaction, but it does not necessarily mean the frequency factor is small; it could also be due to a high activation energy. Thus, the relationship between these factors is crucial for understanding reaction kinetics.
Recommended video:
Guided course
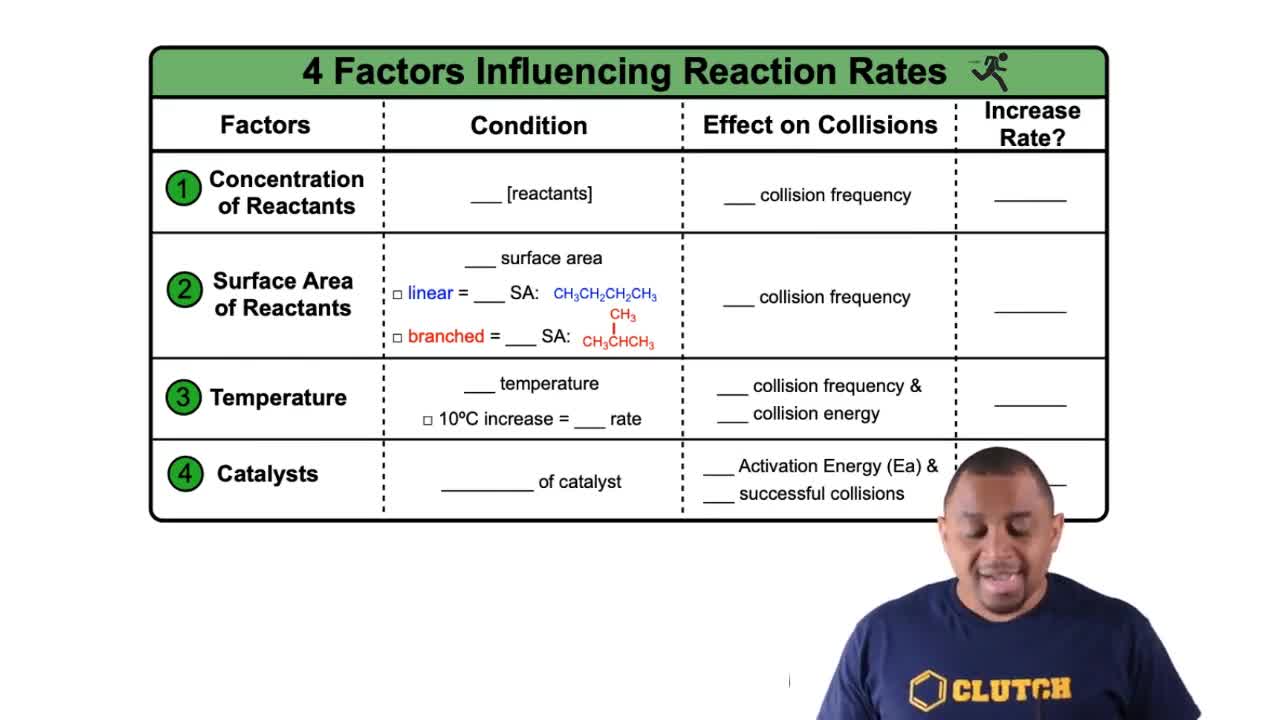
Four Factors Influencing Reaction Rates
Related Practice
Textbook Question
Textbook Question
The gas-phase reaction Cl(g) + HBr(g) → HCl(g) + Br(g) has an overall energy change of -66 kJ. The activation energy for the reaction is 7 kJ. (b) What is the activation energy for the reverse reaction?
Textbook Question
Indicate whether each statement is true or false. (c) Increasing the reaction temperature increases the fraction of successful collisions between reactants.
1
views
Textbook Question
Indicate whether each statement is true or false. (a) If you measure the rate constant for a reaction at different temperatures, you can calculate the overall enthalpy change for the reaction.
2
views
Textbook Question
Indicate whether each statement is true or false. (b) Exothermic reactions are faster than endothermic reactions.
