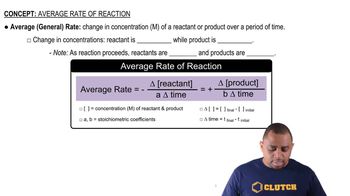
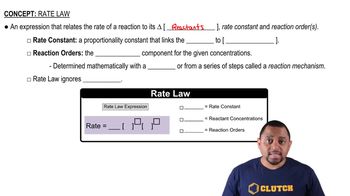
Consider the gas-phase reaction between nitric oxide and bromine at 273°C: 2 NO(g) + Br2(g) → 2 NOBr(g). The following data for the initial rate of appearance of NOBr were obtained:
Experiment [NO] (M) [Br2] (M) Initial Rate (M/s)
1 0.10 0.20 24
2 0.25 0.20 150
3 0.10 0.50 60
4 0.35 0.50 735
(b) Calculate the average value of the rate constant for the appearance of NOBr from the four data sets.