(a) The activation energy for the isomerization of methyl isonitrile (Figure 14.6) is 160 kJ>mol. Calculate the fraction of methyl isonitrile molecules that has an energy equal to or greater than the activation energy at 500 K. (b) Calculate this fraction for a temperature of 520 K. What is the ratio of the fraction at 520 K to that at 500 K?
Ch.14 - Chemical Kinetics

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 14, Problem 52
(a) In which of the following reactions would you expect the orientation factor to be least important in leading to the reaction: NO + O → NO2 or H + Cl → HCl? (b) Does the orientation factor depend on temperature?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the concept of the orientation factor. The orientation factor, also known as the steric factor, is a component of the collision theory of chemical reactions. It accounts for the fact that not all collisions between reactant molecules lead to a reaction, as the molecules must be oriented in a specific way to react.
Step 2: Analyze the molecular structure and complexity of the reactants in each reaction. The orientation factor is generally less important for reactions involving simple diatomic molecules compared to more complex molecules. Consider the molecular complexity of NO + O and H + Cl.
Step 3: Compare the reactions. NO + O involves a diatomic molecule and a single atom, while H + Cl involves two atoms. The reaction involving simpler molecules (H + Cl) is likely to have a less significant orientation factor because there are fewer ways the molecules can be oriented incorrectly.
Step 4: Consider the role of temperature on the orientation factor. The orientation factor is generally considered to be independent of temperature. However, higher temperatures can increase the kinetic energy of molecules, potentially increasing the likelihood of effective collisions.
Step 5: Conclude the analysis. Based on the molecular complexity and the nature of the reactants, determine which reaction has a less significant orientation factor and confirm that the orientation factor is not dependent on temperature.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Orientation Factor
The orientation factor is a term used in collision theory that describes how the spatial arrangement of reactant molecules affects the likelihood of a successful reaction. It accounts for the fact that not all collisions between reactants lead to a reaction; only those with the correct orientation can result in product formation. Reactions involving simple molecules or those with fewer degrees of freedom typically have a lower orientation factor.
Recommended video:
Guided course

Orientation Factor Example
Collision Theory
Collision theory posits that for a reaction to occur, reactant molecules must collide with sufficient energy and proper orientation. The theory emphasizes that both the frequency of collisions and the energy of those collisions are critical for determining reaction rates. Understanding this theory helps in analyzing how different factors, such as molecular complexity and temperature, influence reaction kinetics.
Recommended video:
Guided course
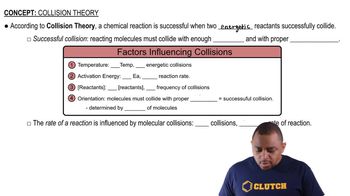
Collision Theory
Temperature Dependence
Temperature affects reaction rates by influencing the kinetic energy of molecules. As temperature increases, molecules move faster, leading to more frequent and energetic collisions. While the orientation factor itself does not directly depend on temperature, the overall reaction rate, which includes the orientation factor, is influenced by temperature changes, as higher temperatures can increase the likelihood of successful collisions.
Recommended video:
Guided course
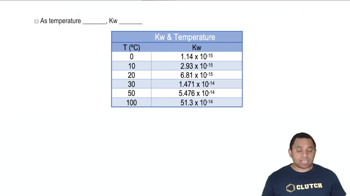
Kw Temperature Dependence
Related Practice
Textbook Question
1
views
Textbook Question
(a) What factors determine whether a collision between two molecules will lead to a chemical reaction?
1
views
Textbook Question
(b) Does the rate constant for a reaction generally increase or decrease with an increase in reaction temperature?
1
views
Textbook Question
Calculate the fraction of atoms in a sample of argon gas at 400 K that has an energy of 10.0 kJ or greater.
Textbook Question
The gas-phase reaction Cl(g) + HBr(g) → HCl(g) + Br(g) has an overall energy change of -66 kJ. The activation energy for the reaction is 7 kJ. (a) Sketch the energy profile for the reaction, and label Ea and ΔE.
