An automotive fuel injector dispenses a fine spray of gasoline into the automobile cylinder, as shown in the bottom drawing here. When an injector gets clogged, as shown in the top drawing, the spray is not as fine or even and the performance of the car declines. How is this observation related to chemical kinetics? [Section 14.1]
Ch.14 - Chemical Kinetics

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 14, Problem 2
A flask is charged with 0.100 mol of A and allowed to react to form B according to the hypothetical gas-phase reaction A(g) → B(g). The following data are collected: Time (s) 0 40 80 120 160 Moles of A 0.100 0.067 0.045 0.030 0.020 (a) Calculate the number of moles of B at each time in the table, assuming that A is cleanly converted to B with no intermediates.
 Verified step by step guidance
Verified step by step guidance1
Identify the initial number of moles of A, which is 0.100 mol. Since the reaction A(g) → B(g) is a 1:1 conversion, the initial moles of B at time 0 is 0 mol.
For each time point, calculate the change in moles of A from the initial amount. For example, at 40 seconds, the moles of A is 0.067 mol, so the change is 0.100 mol - 0.067 mol = 0.033 mol.
Since the reaction is a direct conversion of A to B, the change in moles of A is equal to the moles of B formed. Therefore, at 40 seconds, the moles of B is 0.033 mol.
Repeat the calculation for each time point: at 80 seconds, the moles of A is 0.045 mol, so the change is 0.100 mol - 0.045 mol = 0.055 mol, which is the moles of B formed.
Continue this process for 120 seconds and 160 seconds, using the same method to find the moles of B at each time point by subtracting the moles of A from the initial moles of A.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Stoichiometry
Stoichiometry is the branch of chemistry that deals with the quantitative relationships between the reactants and products in a chemical reaction. In this case, the reaction A(g) → B(g) implies a 1:1 molar ratio, meaning that for every mole of A that reacts, one mole of B is produced. Understanding stoichiometry is essential for calculating the moles of B formed as A is consumed over time.
Recommended video:
Guided course

Stoichiometry Concept
Mole Concept
The mole concept is a fundamental principle in chemistry that quantifies the amount of substance. One mole corresponds to 6.022 x 10²³ entities (atoms, molecules, etc.). In this scenario, knowing the initial amount of A (0.100 mol) allows us to determine how many moles of B are produced at various time intervals by subtracting the remaining moles of A from the initial amount.
Recommended video:
Guided course

Mole Concept
Reaction Kinetics
Reaction kinetics studies the rates of chemical reactions and how they change over time. In this problem, the data collected at different time intervals shows the decrease in moles of A, which can be used to infer the rate of the reaction. Understanding the kinetics helps in predicting how quickly A is converted to B and allows for the calculation of moles of B at each time point.
Recommended video:
Guided course
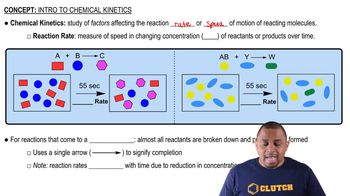
Chemical Kinetics
Related Practice
Textbook Question
1
views
Textbook Question
Consider the following graph of the concentration of a substance X over time. Is each of the following statements true or false? (d) As time progresses, the curve will eventually turn downward toward the x-axis. [Section 14.2]
1
views
Textbook Question
You study the rate of a reaction, measuring both the concentration of the reactant and the concentration of the product as a function of time, and obtain the following results:
Which chemical equation is consistent with these data: (i) A → B, (ii) B → A, (iii) A → 2 B, (iv) B → 2 A?
Textbook Question
Suppose that for the reaction K + L → M, you monitor the production of M over time, and then plot the following graph from your data:
(b) Is the reaction completed at t = 15 min? [Section 14.2]
2
views
