For each of the following gas-phase reactions, indicate how the rate of disappearance of each reactant is related to the rate of appearance of each product: (c) N21g2 + 3 H21g2¡2 NH31g2

(a) Consider the combustion of hydrogen, 2 H21g2 + O21g2 ¡ 2 H2O1g2. If hydrogen is burning at the rate of 0.48 mol>s, what is the rate of consumption of oxygen? What is the rate of formation of water vapor?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
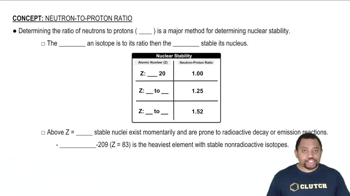
Key Concepts
Stoichiometry

Mole Ratio
Reaction Rates

For each of the following gas-phase reactions, indicate how the rate of disappearance of each reactant is related to the rate of appearance of each product:
(d) C2H5NH2(g) → C2H4(g) + NH3(g)
For each of the following gas-phase reactions, write the rate expression in terms of the appearance of each product and disappearance of each reactant:
(a) 2 H2O(g) → 2 H2(g) + O2(g)
(b) 2 SO2(g) + O2(g) → 2 SO3(g)
(c) 2 NO(g) + 2 H2(g) → N2(g) + 2 H2O(g)
(d) N2(g) + 2 H2(g) → N2H4(g)
(b) The reaction 2 NO1g2 + Cl21g2¡2 NOCl1g2 is carried out in a closed vessel. If the partial pressure of NO is decreasing at the rate of 56 torr/min, what is the rate of change of the total pressure of the vessel?
A reaction A + B → C obeys the following rate law: Rate = k[B]2. (a) If [A] is doubled, how will the rate change? Will the rate constant change?
