Textbook Question
Indicate the principal type of solute–solvent interaction in each of the following solutions and rank the solutions from weakest to strongest solute–solvent interaction: (c) methanol (CH3OH) in water
1
views

 Verified step by step guidance
Verified step by step guidance
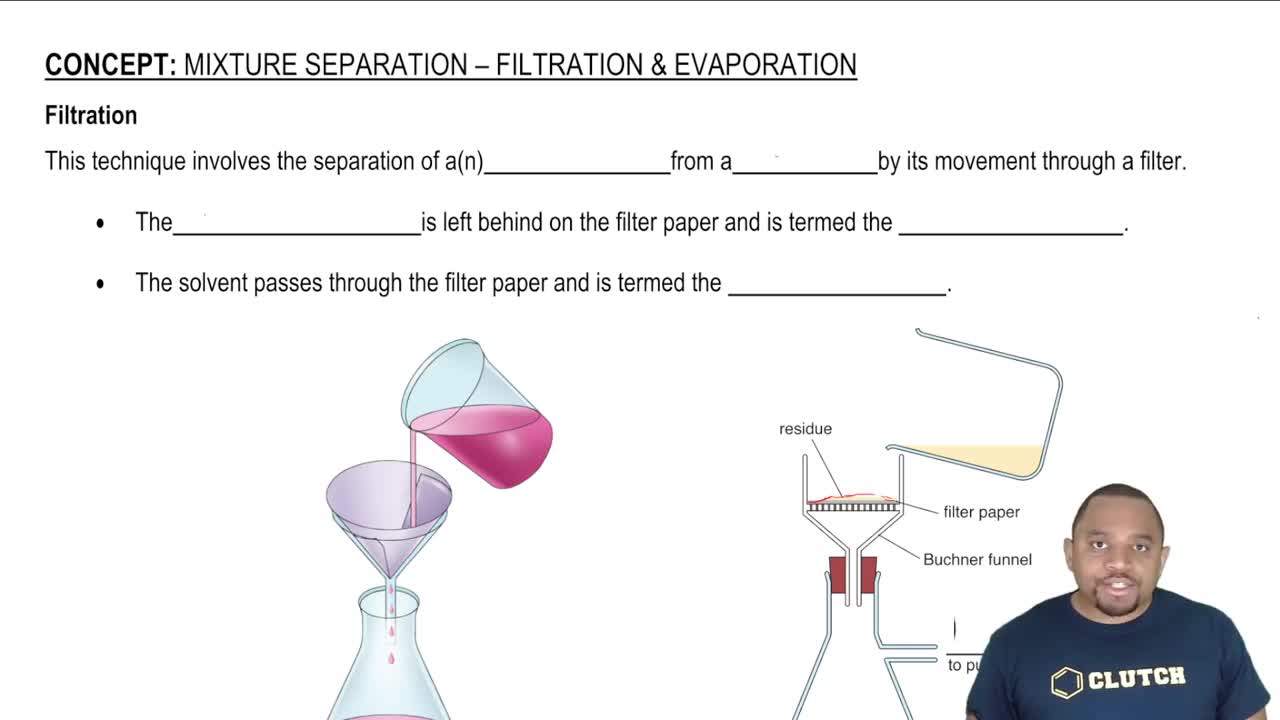

Indicate the principal type of solute–solvent interaction in each of the following solutions and rank the solutions from weakest to strongest solute–solvent interaction: (c) methanol (CH3OH) in water
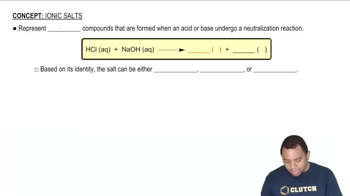
An ionic compound has a very negative ∆Hsoln in water (b) Which term would you expect to be the largest negative number: ∆Hsolvent, ∆Hsolute, or ∆Hmix?
When ammonium chloride dissolves in water, the solution becomes colder. (a) Is the solution process exothermic or endothermic?
When ammonium chloride dissolves in water, the solution becomes colder. (b) Why does the solution form?