Textbook Question
By referring to Figure 13.15, determine the mass of each of the following salts required to form a saturated solution in 250 g of water at 30 °C: (b) Pb(NO3)2,

 Verified step by step guidance
Verified step by step guidance

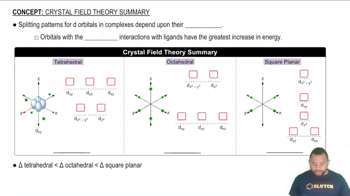

By referring to Figure 13.15, determine the mass of each of the following salts required to form a saturated solution in 250 g of water at 30 °C: (b) Pb(NO3)2,
By referring to Figure 13.15, determine the mass of each of the following salts required to form a saturated solution in 250 g of water at 30 °C: (c) Ce2(SO4)3.
Which of the following in each pair is likely to be more soluble in hexane, C6H14: (a) CCl4 or CaCl2, (b) benzene (C6H6) or glycerol, CH2(OH)CH(OH)CH2OH, (c) octanoic acid, CH3CH2CH2CH2CH2CH2CH2COOH, or acetic acid, CH3COOH? Explain your answer in each case.
Which of the following in each pair is likely to be more soluble in water: (a) cyclohexane (C6H12) or glucose (C6H12O6),