At 20 °C, the vapor pressure of benzene (C6H6) is 75 torr, and that of toluene (C7H8) is 22 torr. Assume that benzene and toluene form an ideal solution. (b) What is the mole fraction of benzene in the vapor above the solution described in part (a)?
Ch.13 - Properties of Solutions

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 13, Problem 72
List the following aqueous solutions in order of decreasing freezing point: 0.040 m glycerin (C3H8O3), 0.020 m KBr, 0.030 m phenol (C6H5OH).
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand that the freezing point of a solution is affected by the number of solute particles in the solution, which is described by the colligative property known as freezing point depression.
Step 2: Recall the formula for freezing point depression: \( \Delta T_f = i \cdot K_f \cdot m \), where \( \Delta T_f \) is the change in freezing point, \( i \) is the van't Hoff factor, \( K_f \) is the freezing point depression constant, and \( m \) is the molality of the solution.
Step 3: Determine the van't Hoff factor \( i \) for each solute: Glycerin (C3H8O3) is a non-electrolyte, so \( i = 1 \); KBr is an electrolyte that dissociates into K+ and Br-, so \( i = 2 \); Phenol (C6H5OH) is a weak electrolyte, but for simplicity, assume \( i = 1 \).
Step 4: Calculate the effective molality for each solution by multiplying the molality by the van't Hoff factor: Glycerin: \( 0.040 \times 1 = 0.040 \), KBr: \( 0.020 \times 2 = 0.040 \), Phenol: \( 0.030 \times 1 = 0.030 \).
Step 5: Rank the solutions in order of decreasing freezing point. The solution with the lowest effective molality will have the highest freezing point. Therefore, the order is: Phenol (0.030 m), Glycerin (0.040 m), KBr (0.040 m).
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Freezing Point Depression
Freezing point depression is a colligative property that describes how the freezing point of a solvent decreases when a solute is added. The extent of this depression depends on the number of solute particles in solution rather than their identity. This means that solutions with more dissolved particles will have lower freezing points.
Recommended video:
Guided course

Freezing Point Depression
Van't Hoff Factor (i)
The Van't Hoff factor (i) indicates the number of particles into which a solute dissociates in solution. For example, KBr dissociates into two ions (K+ and Br-), giving it an i value of 2, while non-electrolytes like glycerin and phenol do not dissociate, so their i values are 1. This factor is crucial for calculating the effective concentration of solute particles affecting freezing point.
Recommended video:
Guided course
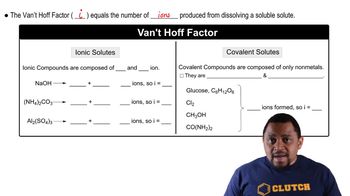
Van't Hoff Factor
Molality (m)
Molality is a measure of concentration defined as the number of moles of solute per kilogram of solvent. It is particularly useful in colligative property calculations because it directly relates to the number of solute particles in a given mass of solvent. In this question, the molalities of the solutions will help determine their respective freezing point depressions.
Recommended video:
Guided course
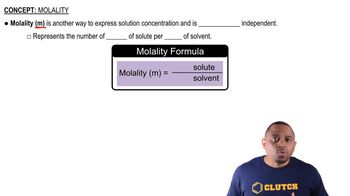
Molality
Related Practice
Textbook Question
Textbook Question
(a) Does a 0.10 m aqueous solution of NaCl have a higher boiling point, a lower boiling point, or the same boiling point as a 0.10 m aqueous solution of C6H12O6?
Textbook Question
List the following aqueous solutions in order of increasing boiling point: 0.120 m glucose, 0.050 m LiBr, 0.050 m Zn(NO3)2.
Textbook Question
Using data from Table 13.3, calculate the freezing and boiling points of each of the following solutions: (a) 0.22 m glycerol (C3H8O3) in ethanol, (b) 0.240 mol of naphthalene (C10H8) in 2.45 mol of chloroform, (c) 1.50 g NaCl in 0.250 kg of water, (d) 2.04 g KBr and 4.82 g of glucose (C6H12O6) in 188 g of water.
Textbook Question
Using data from Table 13.3, calculate the freezing and boiling points of each of the following solutions: (a) 0.25 m glucose in ethanol; (b) 20.0 g of decane, C10H22, in 50.0 g CHCl3; (c) 3.50 g NaOH in 175 g of water, (d) 0.45 mol ethylene glycol and 0.15 mol KBr in 150 g H2O.
1
views
