Indicate the principal type of solute–solvent interaction in each of the following solutions and rank the solutions from weakest to strongest solute–solvent interaction: (a) KCl in water

An ionic compound has a very negative ∆Hsoln in water. (a) Would you expect it to be very soluble or nearly insoluble in water?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Enthalpy of Solution (∆Hsoln)

Ionic Compounds and Solubility

Factors Affecting Solubility
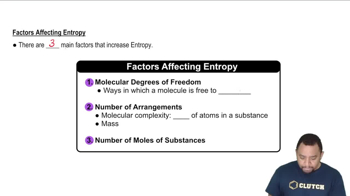
Indicate the principal type of solute–solvent interaction in each of the following solutions and rank the solutions from weakest to strongest solute–solvent interaction: (b) CH2Cl2 in benzene (C6H6)
Indicate the principal type of solute–solvent interaction in each of the following solutions and rank the solutions from weakest to strongest solute–solvent interaction: (c) methanol (CH3OH) in water
An ionic compound has a very negative ∆Hsoln in water (b) Which term would you expect to be the largest negative number: ∆Hsolvent, ∆Hsolute, or ∆Hmix?
The first stage of treatment at the reverse osmosis plant in Carlsbad, California, is to flow the water through rock, sand, and gravel as shown here. Would this step remove particulate matter? Would this step remove dissolved salts?
[Section 18.4]
When ammonium chloride dissolves in water, the solution becomes colder. (a) Is the solution process exothermic or endothermic?
