A particular form of cinnabar (HgS) adopts the zinc blende structure. The length of the unit cell edge is 5.852 Å. (b) The mineral tiemannite (HgSe) also forms a solid phase with the zinc blende structure. The length of the unit cell edge in this mineral is 6.085 Å. What accounts for the larger unit cell length in tiemmanite?

The coordination number for Mg2+ ion is usually six. Assuming this holds, determine the anion coordination number in the following compounds: (a) MgS, (b) MgF2, (c) MgO.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Coordination Number
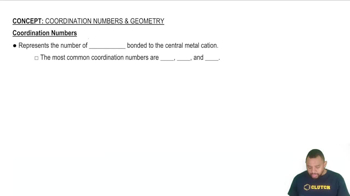
Ionic Compounds

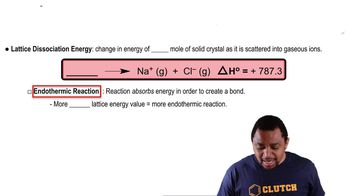
Crystal Lattice Structure
CuI, CsI, and NaI each adopt a different type of structure. The three different structures are those shown in Figure 12.26. (a) Use ionic radii, Cs+ 1r = 1.81 A 2, Na+ 1r = 1.16 A 2, Cu+ 1r = 0.74 A 2, and, I- 1r = 2.06 A 2, to predict which compound will crystallize with which structure.
The coordination number for the Al3+ ion is typically between four and six. Use the anion coordination number to determine the Al3 + coordination number in the following compounds: (a) AlF3 where the fluoride ions are two coordinate.
The coordination number for the Al3+ ion is typically between four and six. Use the anion coordination number to determine the Al3 + coordination number in the following compounds: (b) Al2O3 where the oxygen ions are six coordinate.
The coordination number for the Al3+ ion is typically between four and six. Use the anion coordination number to determine the Al3 + coordination number in the following compounds: (c) AlN where the nitride ions are four coordinate.
