Energy bands are considered continuous due to the large number of closely spaced energy levels. The range of energy levels in a crystal of copper is approximately 1 × 10–19 J. Assuming equal spacing between levels, the spacing between energy levels may be approximated by dividing the range of energies by the number of atoms in the crystal. (b) Determine the average spacing in J between energy levels in the copper metal in part (a).

Teflon is a polymer formed by the polymerization of F2C=CF2. (a) Draw the structure of a section of this polymer. (b) What type of polymerization reaction is required to form Teflon?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Polymerization
Addition Polymerization
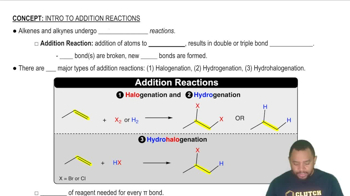
Structure of Teflon

Sodium oxide (Na2O) adopts a cubic structure with Na atoms represented by green spheres and O atoms by red spheres.
(c) The unit cell edge length is 5.550 Å. Determine the density of Na2O.
In their study of X-ray diffraction, William and Lawrence Bragg determined that the relationship among the wavelength of the radiation 1l2, the angle at which the radiation is diffracted 1u2, and the distance between planes of atoms in the crystal that cause the diffraction (d) is given by nl = 2d sin u. X rays from a copper X-ray tube that have a wavelength of 1.54 Å are diffracted at an angle of 14.22 degrees by crystalline silicon. Using the Bragg equation, calculate the distance between the planes of atoms responsible for diffraction in this crystal, assuming n = 1 (first-order diffraction).
