CdS has a band gap of 2.4 eV. If large crystals of CdS are illuminated with ultraviolet light, they emit light equal to the band gap energy. (b) Would appropriately sized CdS quantum dots be able to emit blue light?
Ch.12 - Solids and Modern Materials

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 12, Problem 98
Indicate whether this statement is true or false: If you want a semiconductor that emits blue light, you could either use a material that has a band gap corresponding to the energy of a blue photon or you could use a material that has a smaller band gap but make an appropriately sized nanoparticle of the same material.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the concept of a semiconductor band gap. The band gap is the energy difference between the valence band and the conduction band in a semiconductor. For a semiconductor to emit light, an electron must transition from the conduction band to the valence band, releasing energy in the form of a photon.
Step 2: Determine the energy of a blue photon. Blue light has a wavelength of approximately 450-495 nm. Use the equation E = \frac{hc}{\lambda} to calculate the energy, where h is Planck's constant, c is the speed of light, and \lambda is the wavelength.
Step 3: Consider the first part of the statement. A semiconductor with a band gap equal to the energy of a blue photon will emit blue light when electrons transition from the conduction band to the valence band.
Step 4: Explore the concept of quantum confinement in nanoparticles. When the size of a semiconductor particle is reduced to the nanoscale, the band gap can increase due to quantum confinement effects, potentially allowing a material with a smaller bulk band gap to emit higher energy (shorter wavelength) light.
Step 5: Evaluate the statement. Both approaches mentioned in the statement are theoretically valid for achieving blue light emission: using a material with a band gap corresponding to blue light energy or using quantum confinement in nanoparticles to adjust the band gap.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Band Gap Energy
The band gap energy is the energy difference between the valence band and the conduction band in a semiconductor. It determines the wavelengths of light that a material can absorb or emit. For a semiconductor to emit blue light, its band gap must correspond to the energy of blue photons, which is approximately 2.5 eV.
Recommended video:
Guided course

Intepreting the Band of Stability
Quantum Size Effect
The quantum size effect occurs when the dimensions of a semiconductor are reduced to the nanoscale, leading to quantization of energy levels. This effect can alter the band gap of the material, allowing a semiconductor with a smaller band gap to emit light at higher energies, such as blue light, when formed into nanoparticles.
Recommended video:
Guided course

Photoelectric Effect
Photon Energy and Wavelength
The energy of a photon is inversely related to its wavelength, described by the equation E = hc/λ, where E is energy, h is Planck's constant, c is the speed of light, and λ is the wavelength. Blue light has a short wavelength (around 450 nm), which corresponds to higher energy photons, necessitating a suitable band gap in the semiconductor for effective emission.
Recommended video:
Guided course
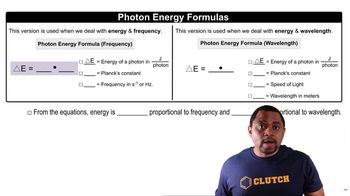
Photon Energy Formulas
Related Practice
Textbook Question
Textbook Question
CdS has a band gap of 2.4 eV. If large crystals of CdS are illuminated with ultraviolet light, they emit light equal to the band gap energy. (c) What about red light?
Textbook Question
Which statement correctly describes a difference between graphene and graphite? (a) Graphene is a molecule but graphite is not. (b) Graphene is a single sheet of carbon atoms and graphite contains many, and larger, sheets of carbon atoms. (c) Graphene is an insulator but graphite is a metal. (d) Graphite is pure carbon but graphene is not. (e) The carbons are sp2 hybridized in graphene but sp3 hybridized in graphite.
