Which type (or types) of crystalline solid is characterized by each of the following? (d) network of covalent bonds.

Indicate the type of solid (molecular, metallic, ionic, or covalent-network) for each compound: (a) InAs, (b) MgO, (c) HgS, (d) In, (e) HBr.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
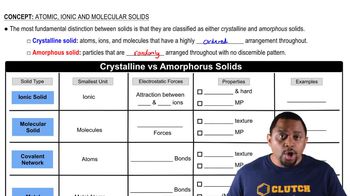
Types of Solids
Ionic Compounds

Metallic and Covalent-Network Solids
Indicate the type of solid (molecular, metallic, ionic, or covalent-network) for each compound: (a) CaSO4 (molecular, metallic, ionic, or covalent-network) for each compound: (b) Pd (molecular, metallic, ionic, or covalent-network) for each compound: (d) caffeine (C8H10N4O2) (molecular, metallic, ionic, or covalent-network) for each compound: (e) toluene (C7H8) (molecular, metallic, ionic, or covalent-network) for each compound: (f) P4.
Indicate the type of solid (molecular, metallic, ionic, or covalent-network) for each compound: (c) Ta2O5 (melting point, 1872°C)
You are given a gray substance that melts at 700 °C; the solid is a conductor of electricity and is insoluble in water. Which type of solid (molecular, metallic, covalent-network, or ionic) might this substance be?
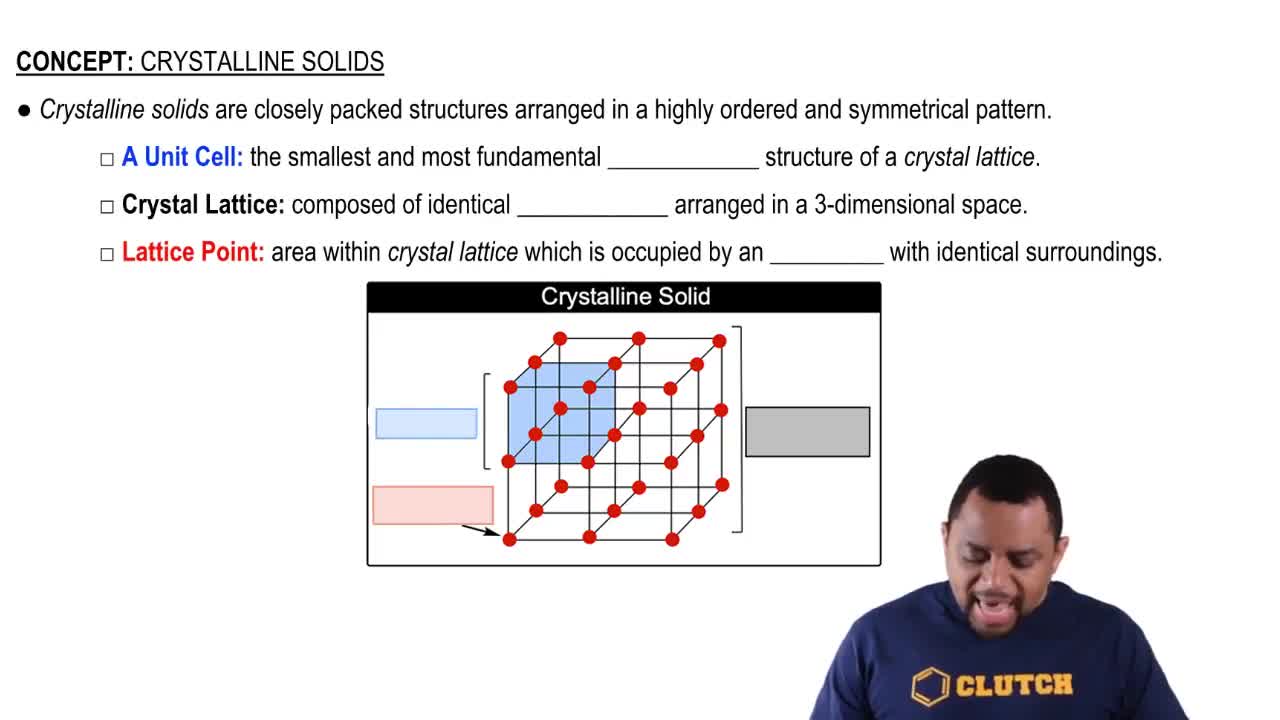
(a) Draw a picture that represents a crystalline solid at the atomic level.
