Textbook Question
CdS has a band gap of 2.4 eV. If large crystals of CdS are illuminated with ultraviolet light, they emit light equal to the band gap energy. (b) Would appropriately sized CdS quantum dots be able to emit blue light?

 Verified step by step guidance
Verified step by step guidance
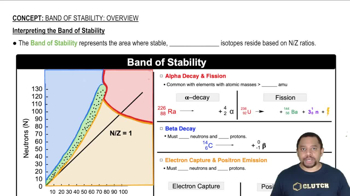

CdS has a band gap of 2.4 eV. If large crystals of CdS are illuminated with ultraviolet light, they emit light equal to the band gap energy. (b) Would appropriately sized CdS quantum dots be able to emit blue light?
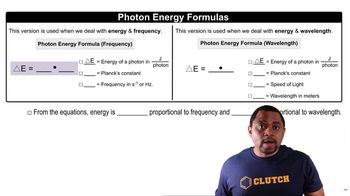
Indicate whether each statement is true or false: (a) Elastomers are rubbery solids. (b) Thermosets cannot be reshaped. (c) Thermoplastic polymers can be recycled.
CdS has a band gap of 2.4 eV. If large crystals of CdS are illuminated with ultraviolet light, they emit light equal to the band gap energy. (c) What about red light?