Textbook Question
(b) What are they in graphite (in one sheet)?


 Verified step by step guidance
Verified step by step guidance

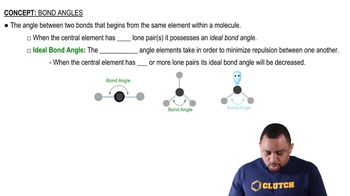

(b) What are they in graphite (in one sheet)?
(c) What atomic orbitals are involved in the stacking of graphite sheets with each other?
Employing the bond enthalpy values listed in Table 8.4, estimate the molar enthalpy change occurring upon (a) polymerization of ethylene. (b) formation of nylon 6,6. (c) formation of polyethylene terephthalate (PET).
(a) In polyvinyl chloride shown in Table 12.6, which bonds have the lowest average bond enthalpy?
(b) When subjected to high pressure and heated, polyvinyl chloride converts to diamond. During this transformation which bonds are most likely to break first?
(c) Employing the values of average bond enthalpy in Table 8.3, estimate the overall enthalpy change for converting PVC to diamond.