You are high up in the mountains and boil water to make some tea. However, when you drink your tea, it is not as hot as it should be. You try again and again, but the water is just not hot enough to make a hot cup of tea. Which is the best explanation for this result? (a) High in the mountains, it is probably very dry, and so the water is rapidly evaporating from your cup and cooling it. (b) High in the mountains, it is probably very windy, and so the water is rapidly evaporating from your cup and cooling it. (c) High in the mountains, the air pressure is significantly less than 1 atm, so the boiling point of water is much lower than at sea level. (d) High in the mountains, the air pressure is significantly less than 1 atm, so the boiling point of water is much higher than at sea level.
Ch.11 - Liquids and Intermolecular Forces

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 11, Problem 56c
Appendix B lists the vapor pressure of water at various external pressures. (c) A city at an altitude of 5000 ft above sea level has a barometric pressure of 633 torr. To what temperature would you have to heat water to boil it in this city?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand that the boiling point of a liquid is the temperature at which its vapor pressure equals the external pressure. In this case, the external pressure is the barometric pressure at the city's altitude, which is 633 torr.
Step 2: Look up the temperature at which the vapor pressure of water is equal to 633 torr in Appendix B. This temperature is the boiling point of water at this altitude.
Step 3: If the exact value of 633 torr is not listed in the table, you may need to interpolate between two values to estimate the boiling point.
Step 4: Interpolation involves finding the two closest values to 633 torr in the table, and estimating the temperature based on these values. For example, if the vapor pressure of water is 600 torr at 90 degrees Celsius and 700 torr at 100 degrees Celsius, you could estimate that the boiling point at 633 torr is somewhere between 90 and 100 degrees Celsius.
Step 5: The exact temperature can be calculated by using the formula for linear interpolation: T = T1 + ((T2 - T1) / (P2 - P1)) * (P - P1), where T is the temperature you're trying to find, T1 and T2 are the temperatures at the two closest pressures, P1 and P2, and P is the pressure you're trying to find (633 torr in this case).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
44sWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Vapor Pressure
Vapor pressure is the pressure exerted by a vapor in equilibrium with its liquid or solid form at a given temperature. It indicates how readily a substance will evaporate; higher vapor pressure means a substance will boil at a lower temperature. Understanding vapor pressure is crucial for determining boiling points under varying external pressures.
Recommended video:
Guided course
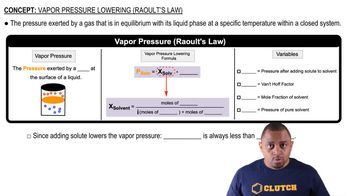
Raoult's Law and Vapor Pressure
Boiling Point
The boiling point of a liquid is the temperature at which its vapor pressure equals the external pressure surrounding the liquid. At higher altitudes, the atmospheric pressure is lower, which decreases the boiling point of water. This concept is essential for calculating the temperature needed to boil water in locations with different barometric pressures.
Recommended video:
Guided course
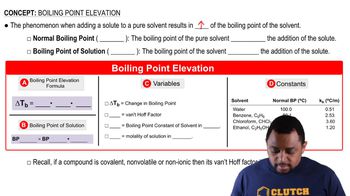
Boiling Point Elevation
Barometric Pressure
Barometric pressure, or atmospheric pressure, is the pressure exerted by the weight of the atmosphere above a given point. It varies with altitude; as altitude increases, barometric pressure decreases. This relationship is important for understanding how boiling points change in different geographical locations, such as cities at high elevations.
Recommended video:
Guided course
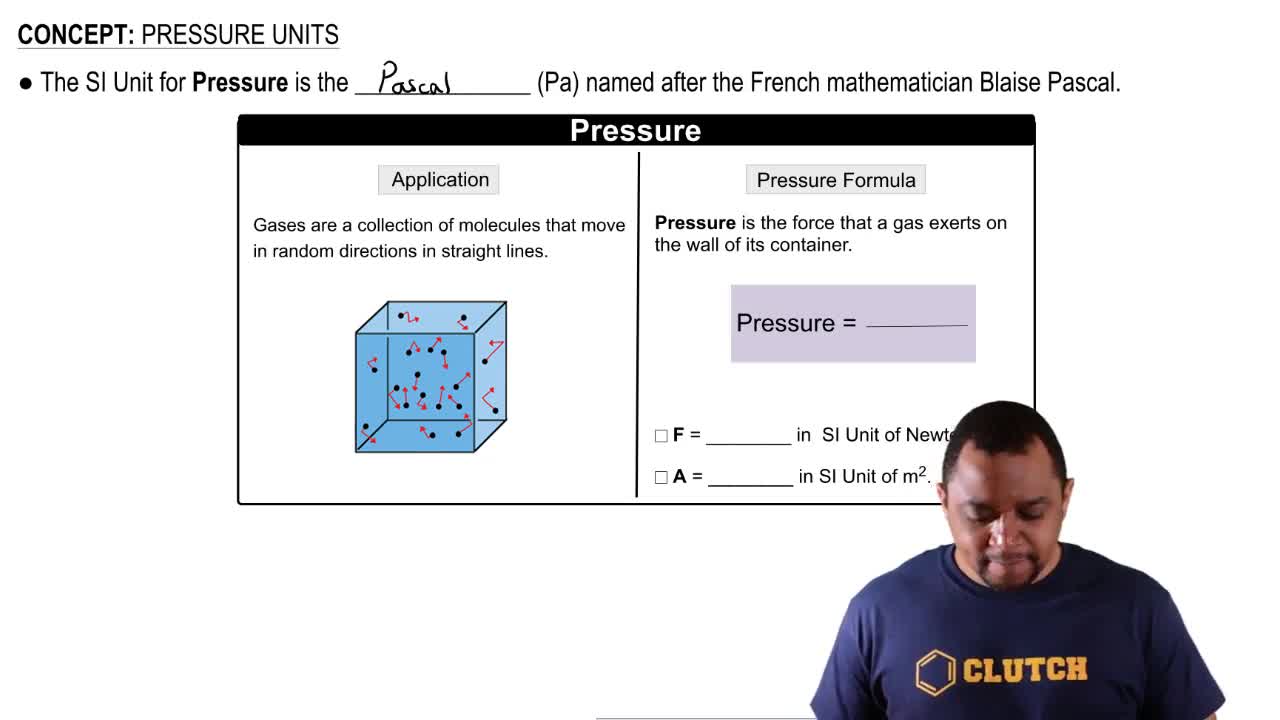
Pressure Units
Related Practice
Textbook Question
1
views
Textbook Question
Using the vapor-pressure curves in Figure 11.25, (d) estimate the external pressure at which diethyl ether will boil at 40 °C.
1
views
Textbook Question
(b) Could you measure the triple point of water by measuring the temperature in a vessel in which water vapor, liquid water, and ice are in equilibrium under 1 atm of air? Explain.
1
views
Textbook Question
Referring to Figure 11.29, describe the phase changes (and the temperatures at which they occur) when CO2 is heated from -80 to -20°C at (a) a constant pressure of 3 atm,
1
views
