Textbook Question
The highest barometric pressure ever recorded was 823.7 torr at Agata in Siberia, Russia on December 31, 1968. Convert this pressure to (b) mm Hg

 Verified step by step guidance
Verified step by step guidance
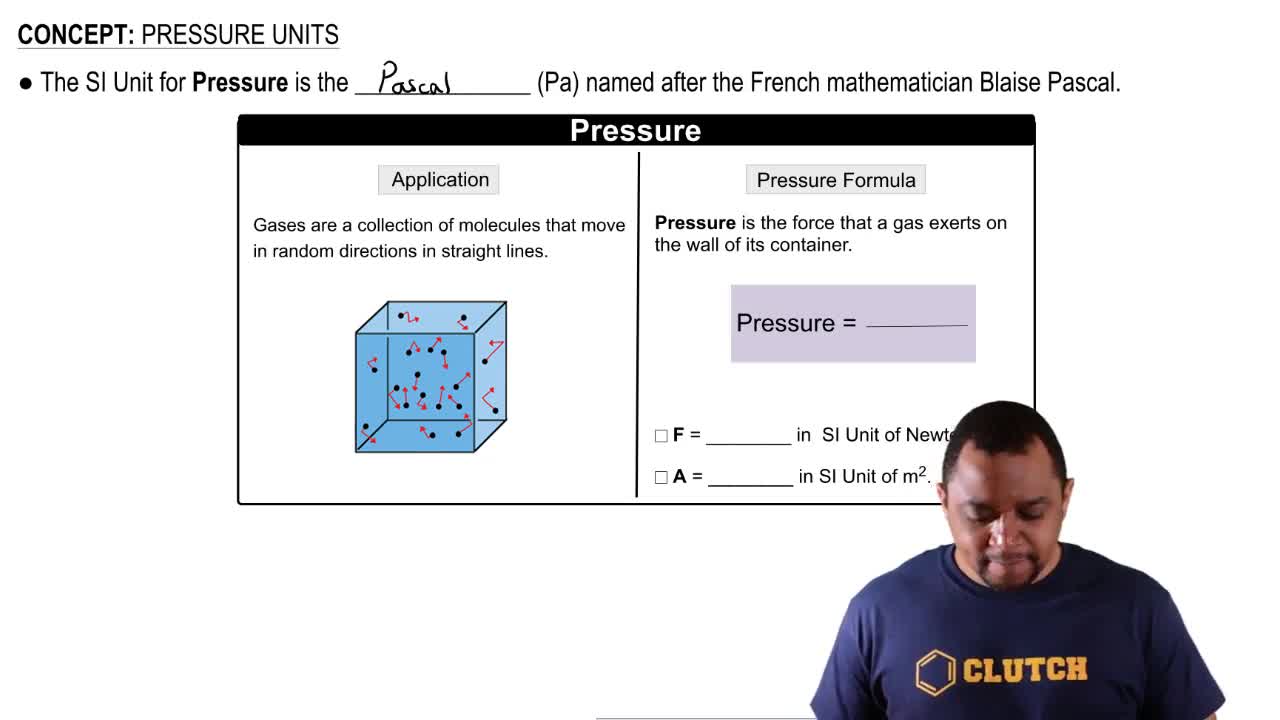


The highest barometric pressure ever recorded was 823.7 torr at Agata in Siberia, Russia on December 31, 1968. Convert this pressure to (b) mm Hg
The highest barometric pressure ever recorded was 823.7 torr at Agata in Siberia, Russia on December 31, 1968. Convert this pressure to (c) pascals.
The highest barometric pressure ever recorded was 823.7 torr at Agata in Siberia, Russia on December 31, 1968. Convert this pressure to (d) bars.
Perform the following conversions: (b) 0.685 bar to kilopascals
Perform the following conversions: (d) 1.323 * 105 Pa to atmospheres