Assume that an exhaled breath of air consists of 74.8% N2, 15.3% O2, 3.7% CO2, and 6.2% water vapor. (b) If the volume of the exhaled gas is 455 mL and its temperature is 37 °C, calculate the number of moles of CO2 exhaled.
Ch.10 - Gases

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 10, Problem 109
An ideal gas at a pressure of 152 kPa is contained in a bulbof unknown volume. A stopcock is used to connect thisbulb with a previously evacuated bulb that has a volumeof 0.800 L as shown here. When the stopcock is opened,the gas expands into the empty bulb. If the temperatureis held constant during this process and the final pressureis 92.66 kPa, what is the volume of the bulb that wasoriginally filled with gas?
 Verified step by step guidance
Verified step by step guidance1
Convert the initial pressure from kPa to atm using the conversion factor: 1 atm = 101.325 kPa.
Use the combined gas law, which states that P1 * V1 = P2 * V2, where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume.
Let V1 be the unknown volume of the initially filled bulb and V2 be the total volume after the stopcock is opened (V1 + 0.800 L).
Substitute the known values into the combined gas law equation: (P1 * V1) = (P2 * (V1 + 0.800 L)).
Solve for V1 by isolating it on one side of the equation.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Ideal Gas Law
The Ideal Gas Law is a fundamental equation in chemistry that relates the pressure (P), volume (V), temperature (T), and number of moles (n) of an ideal gas. It is expressed as PV = nRT, where R is the ideal gas constant. This law allows us to predict the behavior of gases under various conditions, making it essential for solving problems involving gas expansion and compression.
Recommended video:
Guided course

Ideal Gas Law Formula
Boyle's Law
Boyle's Law states that the pressure of a gas is inversely proportional to its volume when the temperature and the amount of gas are held constant. Mathematically, it can be expressed as P1V1 = P2V2. This principle is crucial for understanding how gases behave when they expand or compress, particularly in scenarios like the one described in the question.
Recommended video:
Guided course
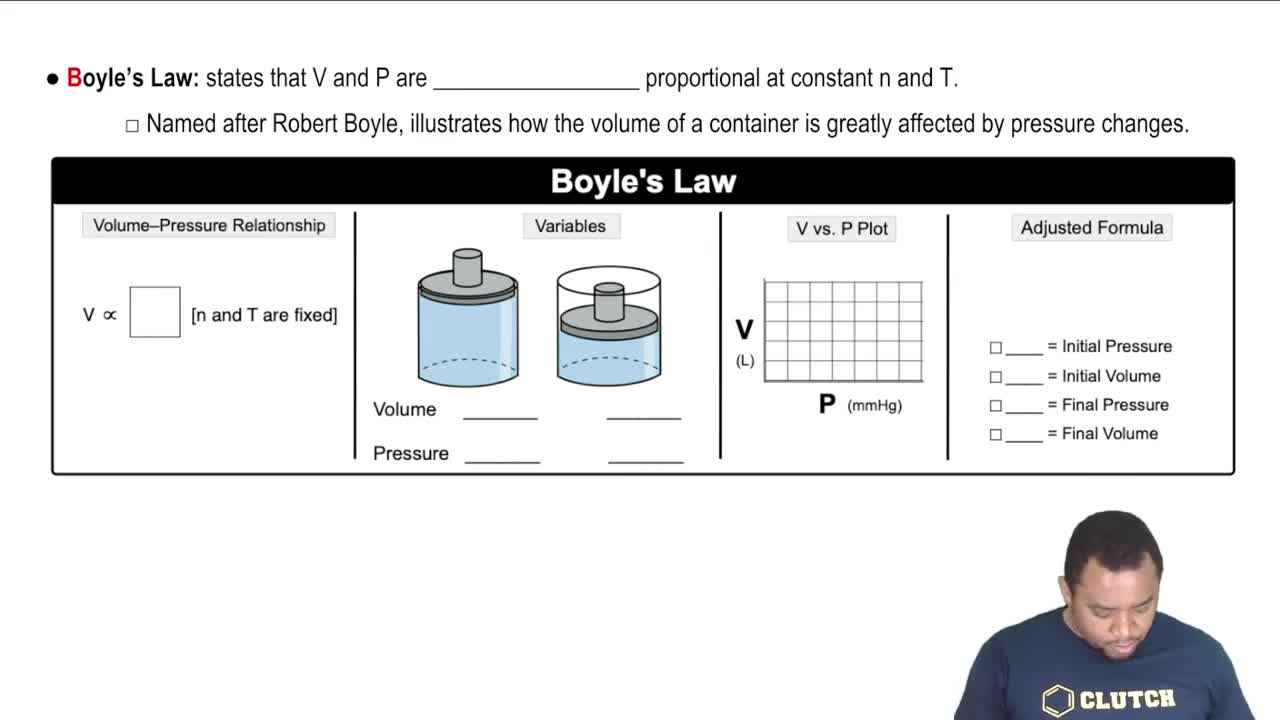
Boyle's Law
Pressure Units and Conversion
Pressure is commonly measured in various units, including atmospheres (atm), pascals (Pa), and kilopascals (kPa). Understanding how to convert between these units is vital for solving gas-related problems. In this question, the initial pressure is given in kPa, while the diagram shows pressure in atm, necessitating conversion for accurate calculations.
Recommended video:
Guided course
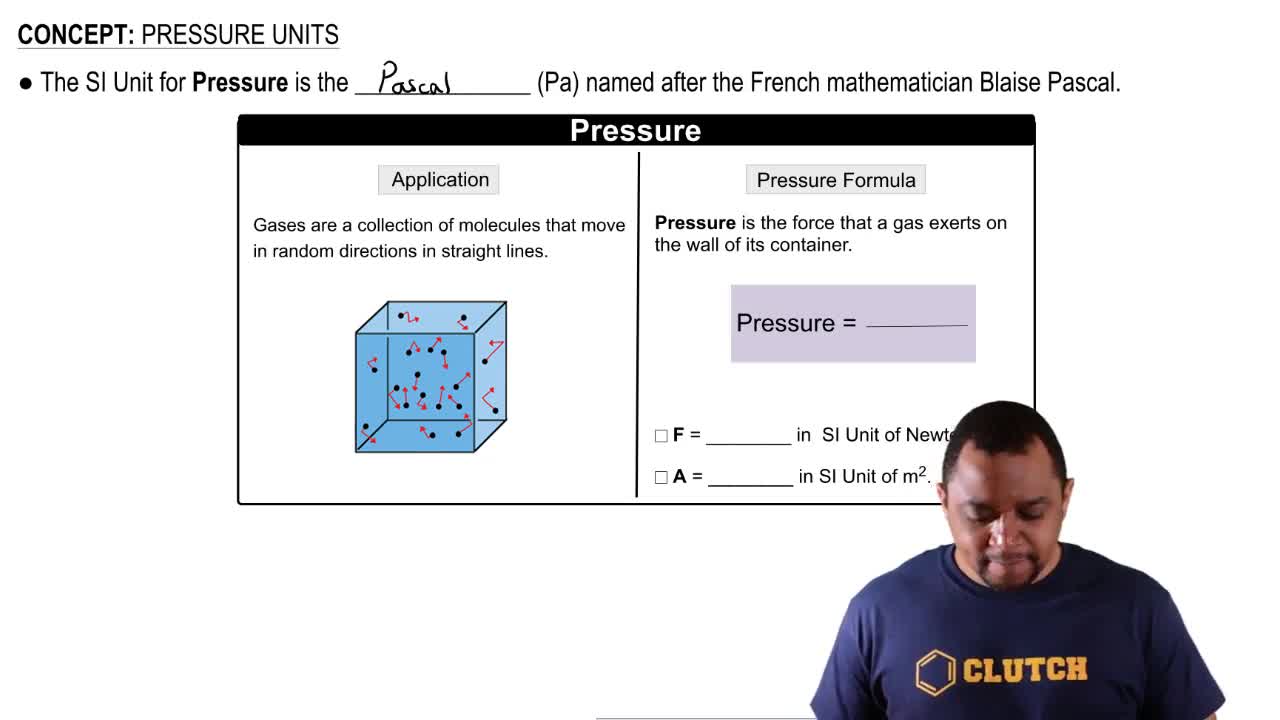
Pressure Units
Related Practice
Textbook Question
Textbook Question
Assume that an exhaled breath of air consists of 74.8% N2, 15.3% O2, 3.7% CO2, and 6.2% water vapor. (c) How many grams of glucose (C6H12O6) would need to be metabolized to produce this quantity of CO2? (The chemical reaction is the same as that for combustion of C6H12O6. See Section 3.2 and Problem 10.57.)
Textbook Question
An 8.40-g sample of argon and an unknown mass of H2are mixed in a flask at room temperature. The partial pressureof the argon is 44.0 kPa, and that of the hydrogen is57.33 kPa. What is the mass of the hydrogen?
Textbook Question
The density of a gas of unknown molar mass was measured as a function of pressure at 0 C, as in the table that follows. (a) Determine a precise molar mass for the gas. [Hint: Graph d>P versus P.]
Textbook Question
The density of a gas of unknown molar mass was measured as a function of pressure at 0 C, as in the table that follows. (b) Why is d>P not a constant as a function of pressure?
