Consider the following graph. (c) For each curve, which speed is highest: the most probable speed, the root-mean-square speed, or the average speed?

A thin glass tube 1 m long is filled with Ar gas at 101.3 kPa, and the ends are stoppered with cotton plugs as shown below. HCl gas is introduced at one end of the tube, and simultaneously NH3 gas is introduced at the other end. When the two gases diffuse through the cotton plugs down the tube and meet, a white ring appears due to the formation of NH4Cl1s2. At which location—a, b, or c—do you expect the ring to form?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
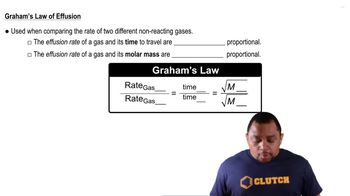
Key Concepts
Graham's Law of Effusion
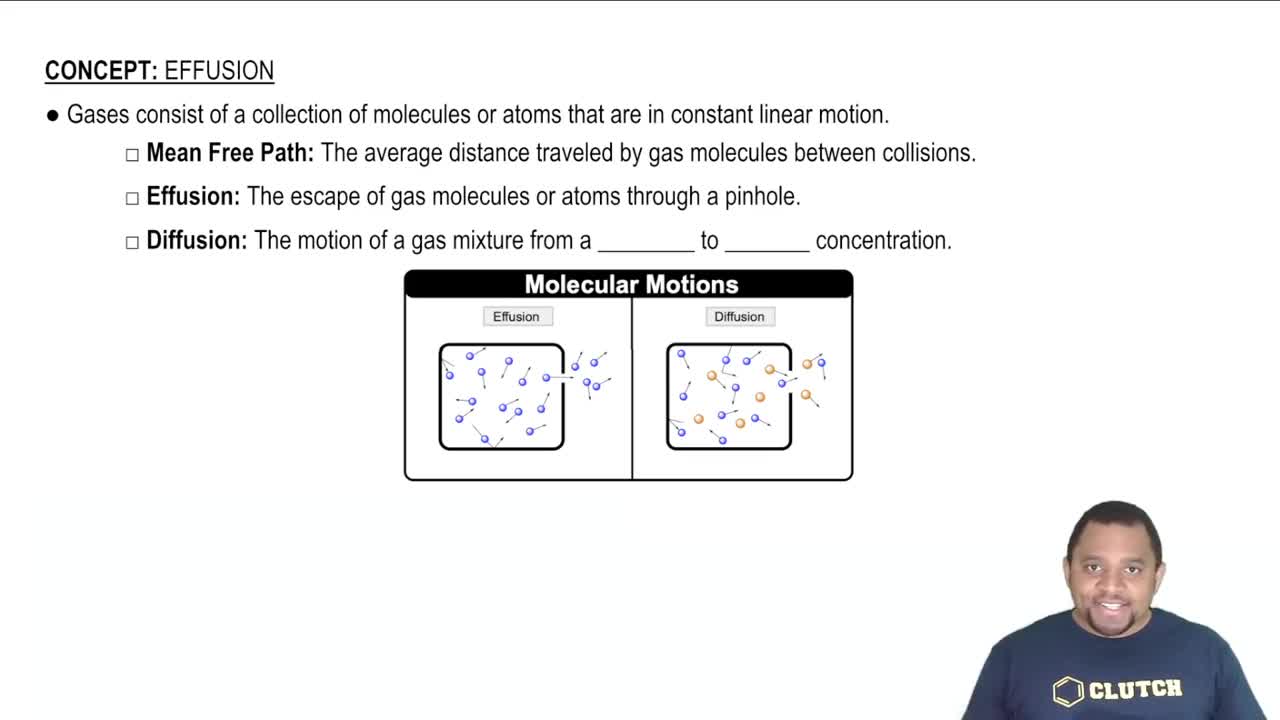
Diffusion
Chemical Reaction and Product Formation

Consider the following samples of gases:
If the three samples are all at the same temperature, rank them with respect to (a) total pressure
Consider the following samples of gases:
If the three samples are all at the same temperature, rank them with respect to (c) density
The graph below shows the change in pressure as the temperature increases for a 1-mol sample of a gas confined to a 1-L container. The four plots correspond to an ideal gas and three real gases: CO2, N2, and Cl2. (a) At room temperature, all three real gases have a pressure less than the ideal gas. Which van der Waals constant, a or b, accounts for the influence intermolecular forces have in lowering the pressure of a real gas?
The graph below shows the change in pressure as the temperature increases for a 1-mol sample of a gas confined to a 1-L container. The four plots correspond to an ideal gas and three real gases: CO2, N2, and Cl2. (b) Use the van der Waals constants in Table 10.3 to match the labels in the plot (A, B, and C) with the respective gases 1CO2, N2, and Cl22.
