Consider the apparatus shown in the following drawing. (a) When the valve between the two containers is opened and the gases are allowed to mix, how does the volume occupied by the N2 gas change?
Ch.10 - Gases

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 10, Problem 63
A mixture containing 0.50 mol H2(g), 1.00 mol O2(g), and 3.50 mol N2(g) is confined in a 25.0-L vessel at 25 °C. Calculate the partial pressure of H2, O2, and N2.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Use the ideal gas law to find the total pressure of the gas mixture. The ideal gas law is given by the equation: \( PV = nRT \), where \( P \) is the pressure, \( V \) is the volume, \( n \) is the number of moles, \( R \) is the ideal gas constant (0.0821 L·atm/mol·K), and \( T \) is the temperature in Kelvin. First, convert the temperature from Celsius to Kelvin by adding 273.15 to the Celsius temperature.
Step 2: Calculate the total number of moles in the mixture by adding the moles of each gas: \( n_{\text{total}} = n_{\text{H}_2} + n_{\text{O}_2} + n_{\text{N}_2} \).
Step 3: Substitute the total number of moles, the volume of the vessel, the temperature in Kelvin, and the ideal gas constant into the ideal gas law equation to solve for the total pressure \( P_{\text{total}} \).
Step 4: Use Dalton's Law of Partial Pressures to find the partial pressure of each gas. According to Dalton's Law, the partial pressure of a gas in a mixture is equal to the mole fraction of that gas multiplied by the total pressure. Calculate the mole fraction of each gas: \( \text{Mole fraction of } H_2 = \frac{n_{\text{H}_2}}{n_{\text{total}}} \), \( \text{Mole fraction of } O_2 = \frac{n_{\text{O}_2}}{n_{\text{total}}} \), \( \text{Mole fraction of } N_2 = \frac{n_{\text{N}_2}}{n_{\text{total}}} \).
Step 5: Calculate the partial pressure of each gas by multiplying its mole fraction by the total pressure: \( P_{\text{H}_2} = \text{Mole fraction of } H_2 \times P_{\text{total}} \), \( P_{\text{O}_2} = \text{Mole fraction of } O_2 \times P_{\text{total}} \), \( P_{\text{N}_2} = \text{Mole fraction of } N_2 \times P_{\text{total}} \).
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Ideal Gas Law
The Ideal Gas Law relates the pressure, volume, temperature, and number of moles of a gas through the equation PV = nRT. This law is essential for calculating the behavior of gases under various conditions, allowing us to determine the pressure exerted by a gas in a mixture when its amount, volume, and temperature are known.
Recommended video:
Guided course

Ideal Gas Law Formula
Partial Pressure
Partial pressure is the pressure that a single gas in a mixture would exert if it occupied the entire volume alone. According to Dalton's Law of Partial Pressures, the total pressure of a gas mixture is the sum of the partial pressures of each individual gas, which can be calculated using the Ideal Gas Law for each component.
Recommended video:
Guided course

Partial Pressure Calculation
Molar Volume of a Gas
At standard temperature and pressure (STP), one mole of an ideal gas occupies approximately 22.4 liters. However, in this scenario, we are using a specific volume (25.0 L) and temperature (25 °C), which requires the use of the Ideal Gas Law to find the partial pressures based on the number of moles and the conditions provided.
Recommended video:
Guided course
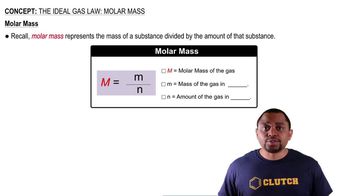
The Ideal Gas Law: Molar Mass
Related Practice
Textbook Question
Textbook Question
Consider the apparatus shown in the following drawing. (a) When the valve between the two containers is opened and the gases are allowed to mix, what is the partial pressure of N2 after mixing?
Textbook Question
Consider a mixture of two gases, A and B, confined in a closed vessel. A quantity of a third gas, C, is added to the same vessel at the same temperature. How does the addition of gas C affect the following: (a) the partial pressure of gas A?
Textbook Question
The atmospheric concentration of CO2 gas is presently 407 ppm (parts per million, by volume; that is, 407 L of every 106 L of the atmosphere are CO2). What is the mole fraction of CO2 in the atmosphere?.
Textbook Question
A plasma-screen TV contains thousands of tiny cells filledwith a mixture of Xe, Ne, and He gases that emits light ofspecific wavelengths when a voltage is applied. A particularplasma cell, 0.900 mm * 0.300 mm * 10.0 mm, contains4% Xe in a 1:1 Ne:He mixture at a total pressure of 66.66 kPa.Calculate the number of Ne atoms in the cell andstate the assumptions you need to make in your calculation.
