Chlorine is widely used to purify municipal water supplies and to treat swimming pool waters. Suppose that the volume of a particular sample of Cl2 gas is 8.70 L at 119.3 kPa and 24 °C. (c) At what temperature will the volume be 15.00 L if the pressure is 116.8 kPa

Many gases are shipped in high-pressure containers. Consider a steel tank whose volume is 210.0 L that contains O2 gas at a pressure of 16,500 kPa at 23 °C. (b) What volume would the gas occupy at STP?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Ideal Gas Law

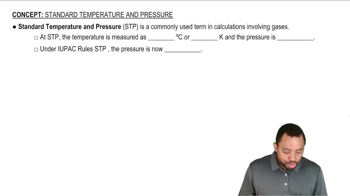
Standard Temperature and Pressure (STP)
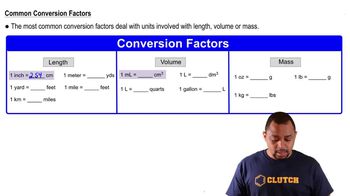
Gas Volume Conversion
Chlorine is widely used to purify municipal water supplies and to treat swimming pool waters. Suppose that the volume of a particular sample of Cl2 gas is 8.70 L at 119.3 kPa and 24 °C. (d) At what pressure will the volume equal 5.00 L if the temperature is 58 °C?
Many gases are shipped in high-pressure containers. Consider a steel tank whose volume is 210.0 L that contains O2 gas at a pressure of 16,500 kPa at 23 °C. (a) What mass of O2 does the tank contain?
Many gases are shipped in high-pressure containers. Consider a steel tank whose volume is 210.0 L that contains O2 gas at a pressure of 16,500 kPa at 23 °C. (c) At what temperature would the pressure in the tank equal 15.2 MPa?
Many gases are shipped in high-pressure containers. Consider a steel tank whose volume is 210.0 L that contains O2 gas at a pressure of 16,500 kPa at 23 °C. (d) What would be the pressure of the gas, in kPa, if it were transferred to a container at 24 °C whose volume is 55.0 L?
