Gold is alloyed (mixed) with other metals to increase its hardness in making jewelry. (a) Consider a piece of gold jewelry that weighs 9.85 g and has a volume of 0.675 cm3. The jewelry contains only gold and silver, which have densities of 19.3 and 10.5 g/cm3, respectively. If the total volume of the jewelry is the sum of the volumes of the gold and silver that it contains, calculate the percentage of gold (by mass) in the jewelry. (b) The relative amount of gold in an alloy is commonly expressed in units of carats. Pure gold is 24 carat, and the percentage of gold in an alloy is given as a percentage of this value. For example, an alloy that is 50% gold is 12 carat. State the purity of the gold jewelry in carats.
Ch.1 - Introduction: Matter, Energy, and Measurement

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 1, Problem 92
Judge the following statements as true or false. If you believe a statement to be false, provide a corrected version. (e) When yellow stains in a kitchen sink are treated with bleach water, the disappearance of the stains is due to a chemical change.
 Verified step by step guidance
Verified step by step guidance1
Understand the nature of chemical changes: A chemical change involves the transformation of substances into new substances with different properties. This often involves breaking and forming chemical bonds.
Consider the role of bleach: Bleach is a chemical that can cause oxidation, a type of chemical reaction where substances lose electrons. This can lead to the breakdown of stains into different compounds.
Evaluate the statement: The disappearance of yellow stains when treated with bleach water suggests that a chemical change has occurred, as the stains are transformed into different substances.
Determine the truth of the statement: Based on the understanding of chemical changes and the action of bleach, the statement is true. The disappearance of stains is due to a chemical change.
If the statement were false, a correction would involve explaining that the disappearance of stains is due to a physical change, but in this case, it is indeed a chemical change.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Chemical Change
A chemical change involves a transformation that alters the chemical composition of a substance, resulting in the formation of new substances. This process is often accompanied by observable changes such as color shifts, gas production, or the release of energy. In the context of the statement, the treatment of stains with bleach can lead to a chemical reaction that breaks down the stain molecules.
Recommended video:
Guided course
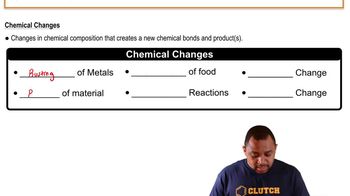
Chemical Changes
Bleaching Agents
Bleaching agents, such as chlorine bleach, are substances that can remove color from materials through oxidation. They work by breaking down the chromophores, which are the parts of molecules responsible for color. Understanding how these agents interact with stains is crucial to determining whether the process is a chemical change or a physical one.
Recommended video:
Guided course
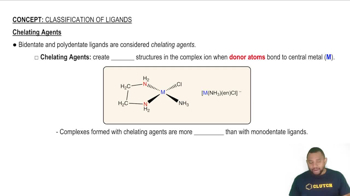
Chelating Agents
Physical Change vs. Chemical Change
A physical change involves alterations in the state or appearance of a substance without changing its chemical identity, such as dissolving or melting. In contrast, a chemical change results in the formation of new substances. Distinguishing between these two types of changes is essential for accurately assessing the nature of the reaction occurring when bleach is applied to stains.
Recommended video:
Guided course
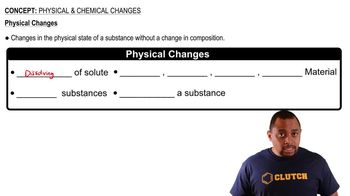
Physical Changes
Related Practice
Textbook Question
Textbook Question
Judge the following statements as true or false. If you believe a statement to be false, provide a corrected version. (a) Air and water are both elements.
36
views
Textbook Question
Judge the following statements as true or false. If you believe a statement to be false, provide a corrected version. (b) All mixtures contain at least one element and one compound.
39
views
Textbook Question
Judge the following statements as true or false. If you believe a statement to be false, provide a corrected version. (c) Compounds can be decomposed into two or more other substances; elements cannot.
47
views
