Classify each of the following as a pure substance or a mixture. If a mixture, indicate whether it is homogeneous or heterogeneous: (c) diamond

 Brown 14th Edition
Brown 14th Edition Ch.1 - Introduction: Matter, Energy, and Measurement
Ch.1 - Introduction: Matter, Energy, and Measurement Problem 15f,g,h,i,j
Problem 15f,g,h,i,jGive the chemical symbol or name for the following elements, as appropriate: (f) Sb (g) Pb (h) Br (i) V (j) Hg.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Chemical Symbols
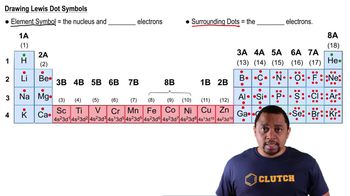
Periodic Table of Elements
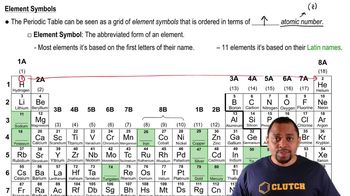
Element Names

Classify each of the following as a pure substance or a mixture. If a mixture, indicate whether it is homogeneous or heterogeneous: (d) mayonnaise.
Give the chemical symbol or name for the following elements, as appropriate: (a) helium (b) platinum (c) cobalt (d) tin (e) silver
Give the chemical symbol or name for each of the following elements, as appropriate: (a) rhenium (b) tungsten (c) cesium (d) hydrogen (e) indium
Give the chemical symbol or name for each of the following elements, as appropriate: (f) As (g) Xe (h) Kr (i) Te (j) Ge.
A solid white substance A is heated strongly in the absence of air. It decomposes to form a new white substance B and a gas C. The gas has exactly the same properties as the product obtained when carbon is burned in an excess of oxygen. Based on these observations, can we determine whether solids A and B and gas C are elements or compounds?