Carbon disulfide (CS2) boils at 46.30 °C and has a density of 1.261 g/mL. (a) When 0.250 mol of a nondissociating solute is dissolved in 400.0 mL of CS2, the solution boils at 47.46 °C. What is the molal boiling-point-elevation constant for CS2?

Carbon disulfide (CS2) boils at 46.30 °C and has a density of 1.261 g/mL. (b) When 5.39 g of a nondissociating unknown is dissolved in 50.0 mL of CS2, the solution boils at 47.08 °C. What is the molar mass of the unknown?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
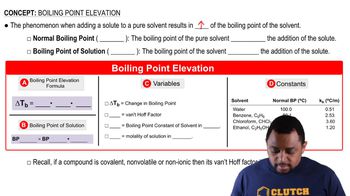
Boiling Point Elevation
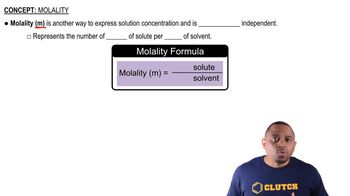
Molality
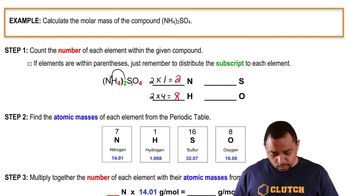
Molar Mass Calculation
Fluorocarbons (compounds that contain both carbon and fluorine) were, until recently, used as refrigerants. The compounds listed in the following table are all gases at 25 °C, and their solubilities in water at 25 °C and 1 atm fluorocarbon pressure are given as mass percentages. (a) For each fluorocarbon, calculate the molality of a saturated solution.
Fluorocarbons (compounds that contain both carbon and fluorine) were, until recently, used as refrigerants. The compounds listed in the following table are all gases at 25 °C, and their solubilities in water at 25 °C and 1 atm fluorocarbon pressure are given as mass percentages. (b) Which molecular property best predicts the solubility of these gases in water: molar mass, dipole moment, or ability to hydrogen-bond to water?
Fluorocarbon Solubility (mass %)
CF4 0.0015
CClF3 0.009
CCl2F2 0.028
CHClF2 0.30
